Polymerized Laminin-521: A Feasible Substrate for Expanding Induced Pluripotent Stem Cells at a Low Protein Concentration
- PMID: 36552719
- PMCID: PMC9777247
- DOI: 10.3390/cells11243955
Polymerized Laminin-521: A Feasible Substrate for Expanding Induced Pluripotent Stem Cells at a Low Protein Concentration
Abstract
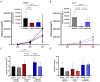
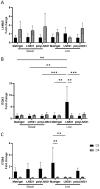
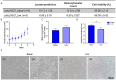
Laminins (LNs) play a central role in the self-assembly and maintenance of basement membranes and are involved in critical interactions between cells and other extracellular matrix (ECM) proteins. Among the defined, xeno-free ECM culture matrices, LNs-namely LN521-have emerged as promising coating systems for the large-scale expansion of induced pluripotent stem cells (iPSCs). The biologic activity of LNs is enhanced by their acidification-induced self-polymerization into a cell-associated network called polylaminin (polyLN), which can recapitulate the native-like polymeric array in a cell-free system. Here, we show for the first time to our knowledge that polyLN521 displays a native-like hexagonal-like structure and that, at basal and low concentrations, it permits the large-scale expansion of human iPSCs. Human iPSCs expanded with polyLN521 maintained the pluripotent state and showed no impairment of karyotype stability or telomere length. These results suggest that low-concentration polyLN521 is a stable and cost-effective coating for large-scale iPSC expansion.
Keywords: cell expansion; iPSC; laminin 521; pluripotency; polylaminin.
Conflict of interest statement
Coelho-Sampaio holds a financial interest in Tatiana Sampaio Servicos de Biologia e Pesquisa Cientifica Eireli. This does not alter the author’s adherence to Biomaterials’ policies on sharing data and materials. All other authors have nothing to disclose regarding commercial support. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Differentiation of human pluripotent stem cells to retinal pigmented epithelium in defined conditions using purified extracellular matrix proteins.J Tissue Eng Regen Med. 2013 Aug;7(8):642-53. doi: 10.1002/term.1458. Epub 2012 Apr 18. J Tissue Eng Regen Med. 2013. PMID: 22514096
-
An extracellular matrix culture system for induced pluripotent stem cells derived from human dental pulp cells.Eur Rev Med Pharmacol Sci. 2015 Nov;19(21):4035-46. Eur Rev Med Pharmacol Sci. 2015. PMID: 26592825
-
A defined xeno-free and feeder-free culture system for the derivation, expansion and direct differentiation of transgene-free patient-specific induced pluripotent stem cells.Biomaterials. 2014 Mar;35(9):2816-26. doi: 10.1016/j.biomaterials.2013.12.050. Epub 2014 Jan 9. Biomaterials. 2014. PMID: 24411336
-
Laminins in Cellular Differentiation.Trends Cell Biol. 2019 Dec;29(12):987-1000. doi: 10.1016/j.tcb.2019.10.001. Epub 2019 Nov 5. Trends Cell Biol. 2019. PMID: 31703844 Review.
-
Laminins in osteogenic differentiation and pluripotency maintenance.Differentiation. 2020 Jul-Aug;114:13-19. doi: 10.1016/j.diff.2020.05.002. Epub 2020 May 16. Differentiation. 2020. PMID: 32473527 Review.
Cited by
-
Laminin Alpha 2 Enhances the Protective Effect of Exosomes on Human iPSC-Derived Cardiomyocytes in an In Vitro Ischemia-Reoxygenation Model.Int J Mol Sci. 2024 Mar 28;25(7):3773. doi: 10.3390/ijms25073773. Int J Mol Sci. 2024. PMID: 38612582 Free PMC article.
-
Wisent Somatic Cells Resist Reprogramming by the PiggyBac Transposon System: A Case Study Highlighting Methodological and Conservation Hurdles.Int J Mol Sci. 2025 May 2;26(9):4327. doi: 10.3390/ijms26094327. Int J Mol Sci. 2025. PMID: 40362564 Free PMC article.
-
Design of dual peptide-conjugated hydrogels for proliferation and differentiation of human pluripotent stem cells.Mater Today Bio. 2024 Jan 23;25:100969. doi: 10.1016/j.mtbio.2024.100969. eCollection 2024 Apr. Mater Today Bio. 2024. PMID: 38318478 Free PMC article.
-
Amniotic fluid collected from vaginal birth as a source of stem cells for clinical applications and disease modeling.Stem Cells Transl Med. 2025 Jun 25;14(7):szaf017. doi: 10.1093/stcltm/szaf017. Stem Cells Transl Med. 2025. PMID: 40558383 Free PMC article.
References
-
- Brehm J.L., Ludwig T.E. Culture, Adaptation, and Expansion of Pluripotent Stem Cells. Methods Mol. Biol. 2017;1590:139–150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

