Development of a Nanoparticle-Based Approach for the Blood-Brain Barrier Passage in a Murine Model of Amyotrophic Lateral Sclerosis
- PMID: 36552768
- PMCID: PMC9776960
- DOI: 10.3390/cells11244003
Development of a Nanoparticle-Based Approach for the Blood-Brain Barrier Passage in a Murine Model of Amyotrophic Lateral Sclerosis
Abstract
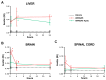
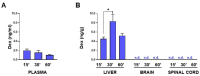
The development of nanoparticles (NPs) to enable the passage of drugs across blood-brain barrier (BBB) represents one of the main challenges in neuropharmacology. In recent years, NPs that are able to transport drugs and interact with brain endothelial cells have been tested. Here, we investigated whether the functionalization of avidin-nucleic-acid-nanoassembly (ANANAS) with apolipoprotein E (ApoE) would allow BBB passage in the SOD1G93A mouse model of amyotrophic lateral sclerosis. Our results demonstrated that ANANAS was able to transiently cross BBB to reach the central nervous system (CNS), and ApoE did not enhance this property. Next, we investigated if ANANAS could improve CNS drug delivery. To this aim, the steroid dexamethasone was covalently linked to ANANAS through an acid-reversible hydrazone bond. Our data showed that the steroid levels in CNS tissues of SOD1G93A mice treated with nanoformulation were below the detection limit. This result demonstrates that the passage of BBB is not sufficient to guarantee the release of the cargo in CNS and that a different strategy for drug tethering should be devised. The present study furthermore highlights that NPs can be useful in improving the passage through biological barriers but may limit the interaction of the therapeutic compound with the specific target.
Keywords: Nanomedicine; amyotrophic lateral sclerosis; blood–brain barrier; pharmacology.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- De Vries H.E., Kooij G., Frenkel D., Georgopoulos S., Monsonego A., Janigro D. Inflammatory Events at Blood-Brain Barrier in Neuroinflammatory and Neurodegenerative Disorders: Implications for Clinical Disease. Epilepsia. 2012;53((Suppl. 6)):45–52. doi: 10.1111/j.1528-1167.2012.03702.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

