Imaging Cataract-Specific Peptides in Human Lenses
- PMID: 36552806
- PMCID: PMC9776990
- DOI: 10.3390/cells11244042
Imaging Cataract-Specific Peptides in Human Lenses
Abstract
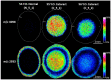
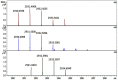
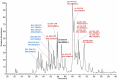
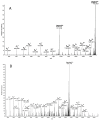
Age-related protein truncation is a common process in long-lived proteins such as proteins found in the ocular lens. Major truncation products have been reported for soluble and membrane proteins of the lens, including small peptides that can accelerate protein aggregation. However, the spatial localization of age-related protein fragments in the lens has received only limited study. Imaging mass spectrometry (IMS) is an ideal tool for examining the spatial localization of protein products in tissues. In this study we used IMS to determine the spatial localization of small crystallin fragments in aged and cataractous lenses. Consistent with previous reports, the pro-aggregatory αA-crystallin 66-80 peptide as well as αA-crystallin 67-80 and γS-crystallin 167-178 were detected in normal lenses, but found to be increased in nuclear cataract regions. In addition, a series of γS-crystallin C-terminal peptides were observed to be mainly localized to cataractous regions and barely detected in transparent lenses. Other peptides, including abundant αA3-crystallin peptides were present in both normal and cataract lenses. The functional properties of these crystallin peptides remain unstudied; however, their cataract-specific localization suggests further studies are warranted.
Keywords: cataract; imaging mass spectrometry; ocular lens; protein degradation.
Conflict of interest statement
The authors have no potential conflict of interest to declare.
Figures
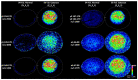
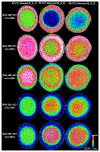
References
-
- Congdon N., Vingerling J.R., Klein B.E.K., West S., Friedman D., Kempen J., O’Colmain B., Wu S.-Y., Taylor H.R. Eye Diseases Prevalence Research Group Prevalence of Cataract and Pseudophakia/Aphakia Among Adults in the United States. Arch. Ophthalmol. 2004;122:487–494. doi: 10.1001/archopht.122.4.487. - DOI - PubMed
-
- Takemoto L. Quantitation of C-terminal modification of alpha-A crystallin during aging of the human lens. Exp. Eye Res. 1995;60:721–724. - PubMed
-
- Wilmarth P.A., Tanner S., Dasari S., Nagalla S.R., Riviere A., Bafna V., Pevzner P.A., David L.L. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: Does deamidation contribute to crystallin insolubility? J. Proteome Res. 2006;5:2554–2566. doi: 10.1021/pr050473a. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

