Cfap91-Dependent Stability of the RS2 and RS3 Base Proteins and Adjacent Inner Dynein Arms in Tetrahymena Cilia
- PMID: 36552811
- PMCID: PMC9776847
- DOI: 10.3390/cells11244048
Cfap91-Dependent Stability of the RS2 and RS3 Base Proteins and Adjacent Inner Dynein Arms in Tetrahymena Cilia
Abstract
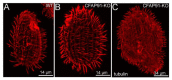
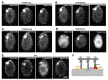
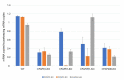
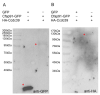
Motile cilia and eukaryotic flagella are specific cell protrusions that are conserved from protists to humans. They are supported by a skeleton composed of uniquely organized microtubules-nine peripheral doublets and two central singlets (9 × 2 + 2). Microtubules also serve as docking sites for periodically distributed multiprotein ciliary complexes. Radial spokes, the T-shaped ciliary complexes, repeat along the outer doublets as triplets and transduce the regulatory signals from the cilium center to the outer doublet-docked dynein arms. Using the genetic, proteomic, and microscopic approaches, we have shown that lack of Tetrahymena Cfap91 protein affects stable docking/positioning of the radial spoke RS3 and the base of RS2, and adjacent inner dynein arms, possibly due to the ability of Cfap91 to interact with a molecular ruler protein, Ccdc39. The localization studies confirmed that the level of RS3-specific proteins, Cfap61 and Cfap251, as well as RS2-associated Cfap206, are significantly diminished in Tetrahymena CFAP91-KO cells. Cilia of Tetrahymena cells with knocked-out CFAP91 beat in an uncoordinated manner and their beating frequency is dramatically reduced. Consequently, CFAP91-KO cells swam about a hundred times slower than wild-type cells. We concluded that Tetrahymena Cfap91 localizes at the base of radial spokes RS2 and RS3 and likely plays a role in the radial spoke(s) positioning and stability.
Keywords: CFAP91; Tetrahymena; axoneme; cilia; radial spoke.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Yuan S., Liu Y., Peng H., Tang C., Hennig G.W., Wang Z., Wang L., Yu T., Klukovich R., Zhang Y., et al. Motile cilia of the male reproductive system require miR-34/miR-449 for development and function to generate luminal turbulence. Proc. Natl. Acad. Sci. USA. 2019;116:3584–3593. doi: 10.1073/pnas.1817018116. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

