Transplantation of Skeletal Muscle-Derived Sca-1+/PW1+/Pax7- Interstitial Cells (PICs) Improves Cardiac Function and Attenuates Remodeling in Mice Subjected to Myocardial Infarction
- PMID: 36552813
- PMCID: PMC9776789
- DOI: 10.3390/cells11244050
Transplantation of Skeletal Muscle-Derived Sca-1+/PW1+/Pax7- Interstitial Cells (PICs) Improves Cardiac Function and Attenuates Remodeling in Mice Subjected to Myocardial Infarction
Erratum in
-
Correction: Ruchaya et al. Transplantation of Skeletal Muscle-Derived Sca-1+/PW1+/Pax7- Interstitial Cells (PICs) Improves Cardiac Function and Attenuates Remodeling in Mice Subjected to Myocardial Infarction. Cells2022, 11, 4050.Cells. 2024 May 23;13(11):895. doi: 10.3390/cells13110895. Cells. 2024. PMID: 38891126 Free PMC article.
Abstract
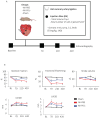
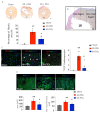
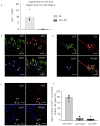
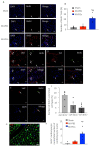
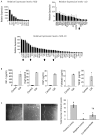
We have previously shown that skeletal muscle-derived Sca-1+/PW1+/Pax7- interstitial cells (PICs) are multi-potent and enhance endogenous repair and regeneration. Here, we investigated the regenerative potential of PICs following intramyocardial transplantation in mice subjected to an acute myocardial infarction (MI). MI was induced through the ligation of the left anterior descending coronary artery in 8-week old male C57BL/6 mice. 5 × 105 eGFP-labelled PICs (MI + PICs; n = 7) or PBS (MI-PBS; n = 7) were injected intramyocardially into the border zone. Sham mice (n = 8) were not subjected to MI, or the transplantation of PICs or PBS. BrdU was administered via osmotic mini-pump for 14 days. Echocardiography was performed prior to surgery (baseline), and 1-, 3- and 6-weeks post-MI and PICs transplantation. Mice were sacrificed at 6 weeks post-MI + PICs transplantation, and heart sections were analysed for fibrosis, hypertrophy, engraftment, proliferation, and differentiation of PICs. A significant (p < 0.05) improvement in ejection fraction (EF) and fractional shortening was observed in the MI-PICs group, compared to MI + PBS group at 6-weeks post MI + PICs transplantation. Infarct size/fibrosis of the left ventricle significantly (p < 0.05) decreased in the MI-PICs group (14.0 ± 2.5%), compared to the MI-PBS group (32.8 ± 2.2%). Cardiomyocyte hypertrophy in the border zone significantly (p < 0.05) decreased in the MI-PICs group compared to the MI-PBS group (330.0 ± 28.5 µM2 vs. 543.5 ± 26.6 µm2), as did cardiomyocyte apoptosis (0.6 ± 0.9% MI-PICs vs. 2.8 ± 0.8% MI-PBS). The number of BrdU+ cardiomyocytes was significantly (p < 0.05) increased in the infarct/border zone of the MI-PICs group (7.0 ± 3.3%), compared to the MI-PBS group (1.7 ± 0.5%). The proliferation index (total BrdU+ cells) was significantly increased in the MI-PICs group compared to the MI-PBS group (27.0 ± 3.4% vs. 7.6 ± 1.0%). PICs expressed and secreted pro-survival and reparative growth factors, supporting a paracrine effect of PICs during recovery/remodeling. Skeletal muscle-derived PICs show significant reparative potential, attenuating cardiac remodelling following transplantation into the infarcted myocardium. PICs can be easily sourced from skeletal muscle and therefore show promise as a potential cell candidate for supporting the reparative and regenerative effects of cell therapies.
Keywords: cardiac repair and regeneration; myocardial infarction; skeletal muscle PW1+/Pax7− interstitial cells (PICs).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Matsuura K., Honda A., Nagai T., Fukushima N., Iwanaga K., Tokunaga M., Shimizu T., Kasanuki H., Hagiwara N., Komuro I. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J. Clin. Investig. 2009;119:2204–2217. doi: 10.1172/JCI37456. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

