Histone Citrullination Mediates a Protective Role in Endothelium and Modulates Inflammation
- PMID: 36552833
- PMCID: PMC9777278
- DOI: 10.3390/cells11244070
Histone Citrullination Mediates a Protective Role in Endothelium and Modulates Inflammation
Abstract
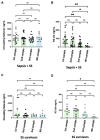
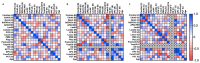
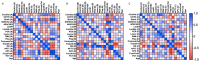
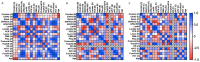
NETosis is a key host immune process against a pathogenic infection during innate immune activation, consisting of a neutrophil "explosion" and, consequently, NET formation, containing mainly DNA, histones, and other nuclear proteins. During sepsis, an exacerbated immune host response to an infection occurs, activating the innate immunity and NETosis events, which requires histone H3 citrullination. Our group compared the circulating histone levels with those citrullinated H3 levels in plasma samples of septic patients. In addition, we demonstrated that citrullinated histones were less cytotoxic for endothelial cells than histones without this post-translational modification. Citrullinated histones did not affect cell viability and did not activate oxidative stress. Nevertheless, citrullinated histones induced an inflammatory response, as well as regulatory endothelial mechanisms. Furthermore, septic patients showed elevated levels of circulating citrullinated histone H3, indicating that the histone citrullination is produced during the first stages of sepsis, probably due to the NETosis process.
Keywords: NETosis; biomarker; citrullination; histones; progression; sepsis; septic shock.
Conflict of interest statement
J.L.G.-G., N.C. and F.V.P. are inventors of a patent (EP3535587B1) related to the detection of circulating histones by mass spectrometry. J.L.G.-G. and F.V.P. are funders and own stocks of EpiDisease S.L., a spin-off from the Consortium Center for Biomedical Network Research (CIBER-ISCIII) and Biomedical Research Institute INCLIVA. The other authors declare no conflicts of interest.
Figures

References
-
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. - DOI - PMC - PubMed
-
- Sakr Y., Jaschinski U., Wittebole X., Szakmany T., Lipman J., Ñamendys-Silva S.A., Martin-Loeches I., Leone M., Lupu M.-N., Vincent J.-L., et al. Sepsis in Intensive Care Unit Patients: Worldwide Data from the Intensive Care over Nations Audit. Open Forum Infect. Dis. 2018;5:ofy313. doi: 10.1093/ofid/ofy313. - DOI - PMC - PubMed
-
- Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

