Recent Advances in the Study of Na+/K+-ATPase in Neurodegenerative Diseases
- PMID: 36552839
- PMCID: PMC9777075
- DOI: 10.3390/cells11244075
Recent Advances in the Study of Na+/K+-ATPase in Neurodegenerative Diseases
Abstract
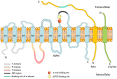
Na+/K+-ATPase (NKA), a large transmembrane protein, is expressed in the plasma membrane of most eukaryotic cells. It maintains resting membrane potential, cell volume and secondary transcellular transport of other ions and neurotransmitters. NKA consumes about half of the ATP molecules in the brain, which makes NKA highly sensitive to energy deficiency. Neurodegenerative diseases (NDDs) are a group of diseases characterized by chronic, progressive and irreversible neuronal loss in specific brain areas. The pathogenesis of NDDs is sophisticated, involving protein misfolding and aggregation, mitochondrial dysfunction and oxidative stress. The protective effect of NKA against NDDs has been emerging gradually in the past few decades. Hence, understanding the role of NKA in NDDs is critical for elucidating the underlying pathophysiology of NDDs and identifying new therapeutic targets. The present review focuses on the recent progress involving different aspects of NKA in cellular homeostasis to present in-depth understanding of this unique protein. Moreover, the essential roles of NKA in NDDs are discussed to provide a platform and bright future for the improvement of clinical research in NDDs.
Keywords: Na+/K+-ATPase; mitochondrial dysfunction; neurodegenerative diseases; oxidative stress; protein–protein interaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Role of Na+/K+-ATPase in ischemic stroke: in-depth perspectives from physiology to pharmacology.J Mol Med (Berl). 2022 Mar;100(3):395-410. doi: 10.1007/s00109-021-02143-6. Epub 2021 Nov 27. J Mol Med (Berl). 2022. PMID: 34839371 Review.
-
Regulation of Neuronal Na+/K+-ATPase by Specific Protein Kinases and Protein Phosphatases.J Neurosci. 2019 Jul 10;39(28):5440-5451. doi: 10.1523/JNEUROSCI.0265-19.2019. Epub 2019 May 13. J Neurosci. 2019. PMID: 31085608 Free PMC article.
-
Anti-Na+/K+-ATPase immunotherapy ameliorates α-synuclein pathology through activation of Na+/K+-ATPase α1-dependent autophagy.Sci Adv. 2021 Jan 27;7(5):eabc5062. doi: 10.1126/sciadv.abc5062. Print 2021 Jan. Sci Adv. 2021. PMID: 33571110 Free PMC article.
-
Tubulin-Na+, K + -ATPase interaction: Involvement in enzymatic regulation and cellular function.J Cell Physiol. 2019 Jun;234(6):7752-7763. doi: 10.1002/jcp.27610. Epub 2018 Oct 30. J Cell Physiol. 2019. PMID: 30378111 Review.
-
Phosphorylation of Na+,K+-ATPase at Tyr10 of the α1-Subunit is Suppressed by AMPK and Enhanced by Ouabain in Cultured Kidney Cells.J Membr Biol. 2021 Dec;254(5-6):531-548. doi: 10.1007/s00232-021-00209-7. Epub 2021 Nov 8. J Membr Biol. 2021. PMID: 34748042 Free PMC article.
Cited by
-
3T sodium-MRI as predictor of neurocognition in nondemented older adults: a cross sectional study.Brain Commun. 2024 Sep 11;6(5):fcae307. doi: 10.1093/braincomms/fcae307. eCollection 2024. Brain Commun. 2024. PMID: 39318783 Free PMC article.
-
Single and Combined Effects of Chlorpyrifos and Glyphosate on the Brain of Common Carp: Based on Biochemical and Molecular Perspective.Int J Mol Sci. 2023 Aug 18;24(16):12934. doi: 10.3390/ijms241612934. Int J Mol Sci. 2023. PMID: 37629125 Free PMC article.
-
The pump, the exchanger, and the Holy Spirit: tracing the 40-year evolution of the Ouabain-Na+ pump endocrine system concept.Ann Med Surg (Lond). 2025 May 30;87(7):4281-4302. doi: 10.1097/MS9.0000000000003438. eCollection 2025 Jul. Ann Med Surg (Lond). 2025. PMID: 40851957 Free PMC article. Review.
-
Oxidative Stress Markers and Na,K-ATPase Enzyme Kinetics Are Altered in the Cerebellum of Zucker Diabetic Fatty fa/fa Rats: A Comparison with Lean fa/+ and Wistar Rats.Biology (Basel). 2024 Sep 25;13(10):759. doi: 10.3390/biology13100759. Biology (Basel). 2024. PMID: 39452068 Free PMC article.
-
Unveiling the role of Na⁺/K⁺-ATPase pump: neurodegenerative mechanisms and therapeutic horizons.Pharmacol Rep. 2025 Jun;77(3):576-592. doi: 10.1007/s43440-025-00717-6. Epub 2025 Mar 21. Pharmacol Rep. 2025. PMID: 40117043 Review.
References
-
- Teixeira F.C., Gutierres J.M., Soares M.S.P., da Siveira de Mattos B., Spohr L., do Couto C.A.T., Bona N.P., Assmann C.E., Morsch V.M., da Cruz I.B.M., et al. Inosine protects against impairment of memory induced by experimental model of Alzheimer disease: A nucleoside with multitarget brain actions. Psychopharmacology. 2020;237:811–823. doi: 10.1007/s00213-019-05419-5. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

