Hepatic Peroxisome Proliferator-Activated Receptor Alpha Dysfunction in Porcine Septic Shock
- PMID: 36552845
- PMCID: PMC9777423
- DOI: 10.3390/cells11244080
Hepatic Peroxisome Proliferator-Activated Receptor Alpha Dysfunction in Porcine Septic Shock
Abstract
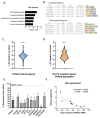
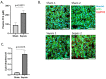
Despite decades of research, sepsis remains one of the most urgent unmet medical needs. Mechanistic investigations into sepsis have mainly focused on targeting inflammatory pathways; however, recent data indicate that sepsis should also be seen as a metabolic disease. Targeting metabolic dysregulations that take place in sepsis might uncover novel therapeutic opportunities. The role of peroxisome proliferator-activated receptor alpha (PPARɑ) in liver dysfunction during sepsis has recently been described, and restoring PPARɑ signaling has proven to be successful in mouse polymicrobial sepsis. To confirm that such therapy might be translated to septic patients, we analyzed metabolic perturbations in the liver of a porcine fecal peritonitis model. Resuscitation with fluids, vasopressor, antimicrobial therapy and abdominal lavage were applied to the pigs in order to mimic human clinical care. By using RNA-seq, we detected downregulated PPARɑ signaling in the livers of septic pigs and that reduced PPARɑ levels correlated well with disease severity. As PPARɑ regulates the expression of many genes involved in fatty acid oxidation, the reduced expression of these target genes, concomitant with increased free fatty acids in plasma and ectopic lipid deposition in the liver, was observed. The results obtained with pigs are in agreement with earlier observations seen in mice and support the potential of targeting defective PPARɑ signaling in clinical research.
Keywords: PPARɑ; free fatty acids; metabolism; sepsis; swine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.v., Ikuta K.S., Kissoon N., Finfer S., et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. - DOI - PMC - PubMed
-
- Evans L., Rhodes A., Alhazzani W., Antonelli M., Coopersmith C.M., French C., Machado F.R., Mcintyre L., Ostermann M., Prescott H.C., et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 2021;47:1181–1247. doi: 10.1007/s00134-021-06506-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

