A Novel Mechanism Underlying the Inhibitory Effects of Trastuzumab on the Growth of HER2-Positive Breast Cancer Cells
- PMID: 36552857
- PMCID: PMC9777316
- DOI: 10.3390/cells11244093
A Novel Mechanism Underlying the Inhibitory Effects of Trastuzumab on the Growth of HER2-Positive Breast Cancer Cells
Abstract
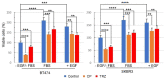
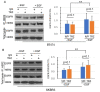
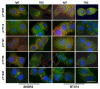
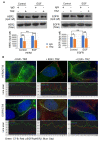
To improve the efficacy of trastuzumab, it is essential to understand its mechanism of action. One of the significant issues that makes it difficult to determine the precise mechanism of trastuzumab action is the formation of various HER receptor dimers in HER2-positive breast cancer cells. So far, studies have focused on the role of HER2-HER3 heterodimers, and little is known regarding EGFR-HER2 heterodimers. Here, we study the role of trastuzumab on the cell signaling and cell proliferation mediated by EGFR-HER2 heterodimers in BT474 and SRBR3 cells. EGF stimulates the formation of both EGFR homodimer and EGFR-HER2 heterodimer. Trastuzumab only binds to HER2, not EGFR. Therefore, any effects of trastuzumab on EGF-induced activation of EGFR, HER2, and downstream signaling proteins, as well as cell proliferation, are through its effects on EGFR-HER2 heterodimers. We show that trastuzumab inhibits EGF-induced cell proliferation and cell cycle progression in BT474 and SKBR3 cells. Interestingly trastuzumab strongly inhibits EGF-induced Akt phosphorylation and slightly inhibits EGF-induced Erk activation, in both BT474 and SKBR3 cells. These data suggest the presence of a novel mechanism that allows trastuzumab to inhibit EGR-induced Akt activation and cell proliferation, without blocking EGF-induced EGFR-HER2 heterodimerization and activation. We show that trastuzumab inhibits EGF-induced lipid raft localization of the EGFR-HER2 heterodimer. Disruption of the lipid raft with MβCD blocks HER2-mediated AKT activation in a similar way to trastuzumab. MβCD and trastuzumab synergically inhibit AKT activation. We conclude that trastuzumab inhibits EGF-induced lipid raft localization of EGFR-HER2 heterodimer, which leads to the inhibition of Akt phosphorylation and cell proliferation, without blocking the formation and phosphorylation of the EGFR-HER2 heterodimer.
Keywords: EGF; EGFR; HER2; akt; cell cycle progression; cell proliferation; dimerization; erk; lipid raft; phosphorylation; trastuzumab.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Modular anti-EGFR and anti-Her2 targeting of SK-BR-3 and BT474 breast cancer cell lines in the presence of ErbB receptor-specific growth factors.Cytometry A. 2011 Sep;79(9):684-93. doi: 10.1002/cyto.a.21107. Epub 2011 Jul 22. Cytometry A. 2011. PMID: 21786419
-
Trastuzumab has preferential activity against breast cancers driven by HER2 homodimers.Cancer Res. 2011 Mar 1;71(5):1871-82. doi: 10.1158/0008-5472.CAN-10-1872. Epub 2011 Feb 15. Cancer Res. 2011. PMID: 21324925 Free PMC article.
-
Combination of antibody that inhibits ligand-independent HER3 dimerization and a p110α inhibitor potently blocks PI3K signaling and growth of HER2+ breast cancers.Cancer Res. 2013 Oct 1;73(19):6013-23. doi: 10.1158/0008-5472.CAN-13-1191. Epub 2013 Aug 5. Cancer Res. 2013. PMID: 23918797 Free PMC article.
-
Mechanism of action of anti-HER2 monoclonal antibodies: scientific update on trastuzumab and 2C4.Adv Exp Med Biol. 2003;532:253-68. doi: 10.1007/978-1-4615-0081-0_21. Adv Exp Med Biol. 2003. PMID: 12908564 Review.
-
Pertuzumab: optimizing HER2 blockade.Clin Cancer Res. 2013 Oct 15;19(20):5552-6. doi: 10.1158/1078-0432.CCR-13-0518. Epub 2013 Aug 13. Clin Cancer Res. 2013. PMID: 23942091 Review.
Cited by
-
Systemic oncological therapy in breast cancer patients on dialysis.World J Clin Oncol. 2024 Jun 24;15(6):730-744. doi: 10.5306/wjco.v15.i6.730. World J Clin Oncol. 2024. PMID: 38946836 Free PMC article. Review.
-
Potential Biomarkers Associated with Prognosis and Trastuzumab Response in HER2+ Breast Cancer.Cancers (Basel). 2023 Sep 1;15(17):4374. doi: 10.3390/cancers15174374. Cancers (Basel). 2023. PMID: 37686651 Free PMC article.
-
Xianling Lianxia formula improves the efficacy of trastuzumab by enhancing NK cell-mediated ADCC in HER2-positive BC.J Pharm Anal. 2024 Oct;14(10):100977. doi: 10.1016/j.jpha.2024.100977. Epub 2024 Apr 10. J Pharm Anal. 2024. PMID: 39493309 Free PMC article.
-
NK cell immunopotentiators-loaded nanoliposomes enhance ADCC effect for targeted therapy against HER2-positive breast cancer.Cell Commun Signal. 2025 Feb 22;23(1):106. doi: 10.1186/s12964-024-02023-9. Cell Commun Signal. 2025. PMID: 39987140 Free PMC article.
-
Molecular Subtypes and Mechanisms of Breast Cancer: Precision Medicine Approaches for Targeted Therapies.Cancers (Basel). 2025 Mar 25;17(7):1102. doi: 10.3390/cancers17071102. Cancers (Basel). 2025. PMID: 40227634 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

