Clinical Performance Evaluation of the NeuMoDx Flu A-B/RSV/SARS-CoV-2 Vantage Assay
- PMID: 36553208
- PMCID: PMC9777658
- DOI: 10.3390/diagnostics12123201
Clinical Performance Evaluation of the NeuMoDx Flu A-B/RSV/SARS-CoV-2 Vantage Assay
Abstract
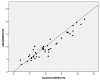
SARS-CoV-2 infections may present with various symptoms that are similar to those of other respiratory diseases. For this reason, the need for simultaneous detection of at least RSV and influenza viruses together with SARS-CoV-2 was evident from the early stages of the pandemic. In the present study, we evaluated the clinical performance of the NeuMoDx™ Flu A-B/RSV/SARS-CoV-2 Vantage Assay against the conventional low-plex PCR utilized to detect influenza A-B, RSV, and SARS-CoV-2. There were 115 known positive clinical samples and 35 negative controls obtained from asymptomatic health-care workers included in the study; 25 samples were positive for influenza viruses, 46 for RSV, and 44 for SARS-CoV-2. The sensitivity, specificity, positive predictive value, and negative predictive value of the evaluated method for influenza and SARS-CoV-2 were 100%. The Spearman correlation coefficient was 0.586 (p < 0.05) for influenza and 0.893 (p < 0.05) for SARS-CoV-2. The sensitivity of the aforementioned assay for RSV was 93.47%; the specificity and the positive predictive value were 100%, and the negative predictive value was 92.10%, while the Spearman correlation coefficient was not applicable for the RSV. Overall, the assay under evaluation was shown to be a reliable alternative for the simultaneous detection of influenza viruses, RSV and SARS-CoV-2.
Keywords: Influenza; RSV; SARS-CoV-2; syndromic testing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Worldometer-Coronavirus. [(accessed on 25 July 2022)]. Available online: https://www.worldometers.info/coronavirus.
-
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—16 March. [(accessed on 25 July 2022)]. Available online: https://www.who.int/director-general/speeches/detail/who-director-genera....
-
- Olsen S.J., Winn A.K., Budd A.P., Prill M.M., Steel J., Midgley C.M., Kniss K., Burns E., Rowe T., Foust A., et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic—United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1013–1019. doi: 10.15585/mmwr.mm7029a1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous