Diagnosing Single and Multiple Drug Hypersensitivity in Children: A Tertiary Care Center Retrospective Study
- PMID: 36553397
- PMCID: PMC9776612
- DOI: 10.3390/children9121954
Diagnosing Single and Multiple Drug Hypersensitivity in Children: A Tertiary Care Center Retrospective Study
Abstract
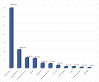
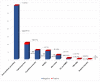
Drug hypersensitivity reactions (DHRs) are a type of adverse drug reactions with heterogeneous pathophysiological mechanisms and a broad spectrum of clinical manifestations. Since over-diagnosing is common in children, a complete allergy work-up is needed. A cross-sectional study was conducted at a tertiary care institution, covering the five-year period. Five hundred and four patients of both sexes, mean age 7.5 and with a medical history suggestive of DHR were evaluated. ENDA/EAACI guidelines were used for a diagnostic algorithm. Single drug hypersensitivity was registered in 375 patients and multiple drug hypersensitivity in 129. The main culprits in medical history were antibiotics (83%), non-steroidal anti-inflammatory drugs (NSAIDs) (8.4%) and analgoantipyretics (3.8%). Skin involvement was registered in 96.2%. DHRs were confirmed in 4.4% patients-six patients had positive skin tests and 13 had a positive drug provocation test. In the proven DHRs group, the main agents were antibiotics (72.7%), followed by NSAIDs (8.3%), and of all the skin manifestations, urticaria was most common (78.2%), followed by exanthema (10.5%) and angioedema (5.3%). Considering the above, anticipating DHRs and a proper referral of children to an allergologist is a key step in the assessment of drug hypersensitivity. A complete allergy work-up prevents unnecessary drug exclusion and allows most children to safely continue the use of first-line medications when needed.
Keywords: allergy diagnostic algorithm; antibiotics; children; cutaneous manifestations; multiple drug hypersensitivity.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Davor Plavec reports grants, personal fees from GlaxoSmithKline; personal fees, non-financial support from Menarini, Philips, and Revenio; personal fees from Pliva, Boehringer Ingelheim, Belupo, Novartis, MSD, and Chiesi, not related to the submitted paper. Asist. Zoran Lekovic reports personal fees from AbbVie, Kibid and New Med, not related to the submitted paper. Aleksa Lekovic and Zoran Lekovic are close relatives. The authors report no other conflicts of interest.
Figures
References
-
- Gomes E.R., Brockow K., Kuyucu S., Saretta F., Mori F., Blancalopez N., Ott H., Atanaskovicmarkovic M., Kidon M.I., Caubet J.-C.R.J.-P., et al. Drug hypersensitivity in children: Report from the pediatric task force of the EAACI Drug Allergy Interest Group. Allergy: European J. Allergy Clin. Immunol. 2016;71:149–161. doi: 10.1111/all.12774. - DOI - PubMed
LinkOut - more resources
Full Text Sources

