Trajectories of Kidney Function in Patients with ATTRv Treated with Gene Silencers
- PMID: 36553503
- PMCID: PMC9777815
- DOI: 10.3390/genes13122236
Trajectories of Kidney Function in Patients with ATTRv Treated with Gene Silencers
Abstract
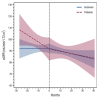
Hereditary transthyretin amyloidosis (ATTRv; v for "variant") is the most common form of hereditary amyloidosis, with an autosomal dominant inheritance and a variable penetrance. This disease has a significant variability in clinical presentation and multiorgan involvement. While kidney involvement in early-onset ATTRv has been reported in one-third of patients, in late-onset ATTRv it has generally been considered rare. In the present study, we describe trajectories of kidney function over time before and after treatment with gene silencing therapies in a cohort of 17 ATTRv patients with different mutations, coming from Italy (nine subjects treated with inotersen and eight patients treated with patisiran). The analysis of estimated glomerular filtration rate (eGFR) slopes revealed that the average change in eGFR was 0.01 mL/min/1.73 m2 per month before initiation and -0.23 mL/min/1.73 m2 per month during follow-up for inotersen and -0.62 mL/min/1.73 m2 per month before initiation and -0.20 mL/min/1.73 m2 per month during follow-up for patisiran. In conclusion, we did not observe any significant difference either between the two groups of treatment or within-group before and after therapy, so gene-silencing therapies may be considered safe for renal function in ATTRv and are not associated with a worsening of eGFR slope.
Keywords: ATTRv; amyloidosis; gene-silencing therapies; kidney involvement.
Conflict of interest statement
Luigetti received financial grants (honoraria and speaking) from Ackea, Alnylam, Sobi, and Pfizer, as well as travel grants from Ackea, Alnylam, Sobi, Pfizer, Kedrion, and Grifols; Romano received financial grants (honoraria and speaking) from Akcea, as well as travel grants from Akcea, Alnylam, Pfizer, and Csl Behring. Guglielmino, Sciarrone, Vitali, and D’Ambrosio have no potential conflicts of interest to be disclosed. Ferraro received grants/consultant fees from Allena Pharmaceuticals, Alnylam, Amgen, AstraZeneca, BioHealth Italia, Gilead, Otsuka Pharmaceuticals, and Vifor Fresenius; Ferraro and D’Ambrosio are members of the European Reference Network for Rare Kidney Diseases (ERKNet)—Project ID N° 739532. Luigetti and Romano are members of the European Reference Network for Neuromuscular Diseases—Project ID N° 870177.
Figures
Similar articles
-
Hereditary Transthyretin Amyloidosis and the Impact of Classic and New Treatments on Kidney Function: A Review.Am J Kidney Dis. 2024 Aug;84(2):224-231. doi: 10.1053/j.ajkd.2024.01.527. Epub 2024 Mar 12. Am J Kidney Dis. 2024. PMID: 38484868 Review.
-
Real-life experience with inotersen in hereditary transthyretin amyloidosis with late-onset phenotype: Data from an early-access program in Italy.Eur J Neurol. 2022 Jul;29(7):2148-2155. doi: 10.1111/ene.15325. Epub 2022 Mar 28. Eur J Neurol. 2022. PMID: 35289020 Free PMC article.
-
Natural course and determinants of short-term kidney function decline in hereditary transthyretin amyloidosis: a French observational study.Amyloid. 2023 Mar;30(1):38-48. doi: 10.1080/13506129.2022.2098011. Epub 2022 Jul 17. Amyloid. 2023. PMID: 35848215
-
Novel approaches to diagnosis and management of hereditary transthyretin amyloidosis.J Neurol Neurosurg Psychiatry. 2022 Jun;93(6):668-678. doi: 10.1136/jnnp-2021-327909. Epub 2022 Mar 7. J Neurol Neurosurg Psychiatry. 2022. PMID: 35256455 Free PMC article. Review.
-
[Gene therapy options for hereditary transthyretin-related amyloidosis].Nervenarzt. 2022 Jun;93(6):557-565. doi: 10.1007/s00115-022-01288-0. Epub 2022 Apr 13. Nervenarzt. 2022. PMID: 35419654 Review. German.
Cited by
-
Emerging multisystem biomarkers in hereditary transthyretin amyloidosis: a pilot study.Sci Rep. 2024 Aug 7;14(1):18281. doi: 10.1038/s41598-024-69123-x. Sci Rep. 2024. PMID: 39112608 Free PMC article.
-
Examining the Difficulties in Identifying and Handling Cardiac Amyloidosis; Acquiring Important Knowledge and Robust Treatment Methods.Cardiovasc Hematol Disord Drug Targets. 2024;24(2):65-82. doi: 10.2174/011871529X301954240715041558. Cardiovasc Hematol Disord Drug Targets. 2024. PMID: 39075963 Review.
-
Kidney Outcomes in Patients With Hereditary Transthyretin Amyloid Nephropathy Treated With Transthyretin Stabilizers And Gene-Silencer Therapies.Kidney Int Rep. 2025 Mar 24;10(6):1939-1949. doi: 10.1016/j.ekir.2025.03.029. eCollection 2025 Jun. Kidney Int Rep. 2025. PMID: 40630302 Free PMC article.
References
-
- Luigetti M., Tortora A., Romano A., Di Paolantonio A., Guglielmino V., Bisogni G., Gasbarrini A., Calabresi P., Sabatelli M. Gastrointestinal Manifestations in Hereditary Transthyretin Amyloidosis: A Single-Centre Experience. J. Gastrointest. Liver Dis. 2020;29:339–343. doi: 10.15403/jgld-2474. - DOI - PubMed
-
- Luigetti M., Conte A., del Grande A., Bisogni G., Madia F., Monaco M.L., Laurenti L., Obici L., Merlini G., Sabatelli M. TTR-related amyloid neuropathy: Clinical, electrophysiological and pathological findings in 15 unrelated patients. Neurol. Sci. 2013;34:1057–1063. doi: 10.1007/s10072-012-1105-y. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

