Whole-Chromosome Karyotyping of Fetal Nucleated Red Blood Cells Using the Ion Proton Sequencing Platform
- PMID: 36553524
- PMCID: PMC9778445
- DOI: 10.3390/genes13122257
Whole-Chromosome Karyotyping of Fetal Nucleated Red Blood Cells Using the Ion Proton Sequencing Platform
Abstract
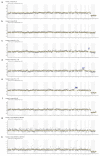
The current gold standard for the definitive diagnosis of fetal aneuploidy uses either chorionic villus sampling (CVS) or amniocentesis, both of which are which are invasive procedures carrying a procedure-related risk of miscarriage of up to 0.1-0.2%. Non-invasive prenatal diagnosis using fetal nucleated red blood cells (FNRBCs) isolated from maternal peripheral venous blood would remove this risk of miscarriage since these cells can be isolated from the mother's blood. We aimed to detect whole-chromosome aneuploidies from single nucleated fetal red blood cells using whole-genome amplification followed by massively parallel sequencing performed on a semiconductor sequencing platform. Twenty-six single cells were picked from the placental villi of twelve patients thought to have a normal fetal genotype and who were undergoing elective first-trimester surgical termination of pregnancy. Following karyotyping, it was subsequently found that two of these cases were also abnormal (one trisomy 15 and one mosaic genotype). One single cell from chorionic villus samples for two patients carrying a fetus with trisomy 21 and two single cells from women carrying fetuses with T18 were also picked. Pooled libraries were sequenced on the Ion Proton and data were analysed using Ion Reporter software. We correctly classified fetal genotype in all 24 normal cells, as well as the 2 T21 cells, the 2 T18 cells, and the two T15 cells. The two cells picked from the fetus with a mosaic result by CVS were classified as unaffected, suggesting that this was a case of confined placental mosaicism. Fetal sex was correctly assigned in all cases. We demonstrated that semiconductor sequencing using commercially available software for data analysis can be achieved for the non-invasive prenatal diagnosis of whole-chromosome aneuploidy with 100% accuracy.
Keywords: aneuploidy; cell based; fetal erythroblast; non-invasive prenatal diagnosis; semiconductor sequencing; single-cell whole-genome sequencing.
Conflict of interest statement
The National University of Singapore has licensed a patent out to INEX Innovate Pte Ltd. MC holds shares in and is a nonexecutive director for INEX Innovate Pte Ltd.; AB is on the scientific advisory board; and SSYH and SA are employees of iGene Laboratory Pte Ltd., which is a subsidiary of INEX Innovate Pte Ltd. No financial benefit was associated with the conduct of this study. None of the other authors have conflicts of interest to declare.
Figures
References
-
- Van der Meij K.R.M., Sistermans E.A., Macville M.V., Stevens S.J., Bax C.J., Bekker M.N., Bilardo C.M., Boon E.M., Boter M., Diderich K.E., et al. TRIDENT-2: National Implementation of Genome-wide Non-invasive Prenatal Testing as a First-Tier Screening Test in the Netherlands. Am. J. Hum. Genet. 2019;105:1091–1101. doi: 10.1016/j.ajhg.2019.10.005. - DOI - PMC - PubMed
-
- Kagan K.O., Maier V., Sonek J., Abele H., Lüthgens K., Schmid M., Wagner P., Hoopmann M. False-Positive Rate in First-Trimester Screening Based on Ultrasound and Cell-Free DNA versus First-Trimester Combined Screening with Additional Ultrasound Markers. Fetal Diagn. Ther. 2019;45:317–324. doi: 10.1159/000489121. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

