The Roles of SNHG Family in Osteoblast Differentiation
- PMID: 36553535
- PMCID: PMC9777675
- DOI: 10.3390/genes13122268
The Roles of SNHG Family in Osteoblast Differentiation
Abstract
Small nucleolar RNA host genes (SNHGs), members of long-chain noncoding RNAs (lncRNAs), have received increasing attention regarding their roles in multiple bone diseases. Studies have revealed that SNHGs display unique expression profile during osteoblast differentiation and that they could act as promising biomarkers of certain bone diseases, such as osteoporosis. Osteogenesis of mesenchymal stem cells (MSCs) is an important part of bone repair and reconstruction. Moreover, studies confirmed that the SNHG family participate in the regulation of osteogenic differentiation of MSCs in part by regulating important pathways of osteogenesis, such as Wnt/β-catenin signaling. Based on these observations, clarifying the SNHG family's roles in osteogenesis (especially in MSCs) and their related mechanisms would provide novel ideas for possible applications of lncRNAs in the diagnosis and treatment of bone diseases. After searching, screening, browsing and intensive reading, we uncovered more than 30 papers related to the SNHG family and osteoblast differentiation that were published in recent years. Here, our review aims to summarize these findings in order to provide a theoretical basis for further research.
Keywords: SNHG family; bone diseases; mesenchymal stem cells; osteoblast differentiation.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
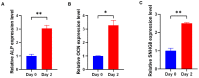

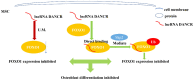
References
-
- Hutchings G., Moncrieff L., Dompe C., Janowicz K., Sibiak R., Bryja A., Jankowski M., Mozdziak P., Bukowska D., Antosik P., et al. Bone Regeneration, Reconstruction and Use of Osteogenic Cells; from Basic Knowledge, Animal Models to Clinical Trials. J. Clin. Med. 2020;9:139. doi: 10.3390/jcm9010139. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

