Challenges in Gene Therapy for Somatic Reverted Mosaicism in X-Linked Combined Immunodeficiency by CRISPR/Cas9 and Prime Editing
- PMID: 36553615
- PMCID: PMC9777626
- DOI: 10.3390/genes13122348
Challenges in Gene Therapy for Somatic Reverted Mosaicism in X-Linked Combined Immunodeficiency by CRISPR/Cas9 and Prime Editing
Abstract
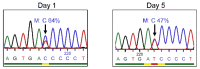
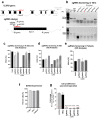
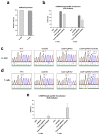
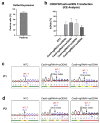
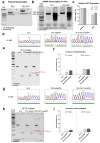
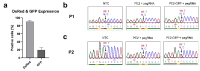
X-linked severe combined immunodeficiency (X-SCID) is a primary immunodeficiency that is caused by mutations in the interleukin-2 receptor gamma (IL2RG) gene. Some patients present atypical X-SCID with mild clinical symptoms due to somatic revertant mosaicism. CRISPR/Cas9 and prime editing are two advanced genome editing tools that paved the way for treating immune deficiency diseases. Prime editing overcomes the limitations of the CRISPR/Cas9 system, as it does not need to induce double-strand breaks (DSBs) or exogenous donor DNA templates to modify the genome. Here, we applied CRISPR/Cas9 with single-stranded oligodeoxynucleotides (ssODNs) and prime editing methods to generate an in vitro model of the disease in K-562 cells and healthy donors' T cells for the c. 458T>C point mutation in the IL2RG gene, which also resulted in a useful way to optimize the gene correction approach for subsequent experiments in patients' cells. Both methods proved to be successful and were able to induce the mutation of up to 31% of treated K-562 cells and 26% of treated T cells. We also applied similar strategies to correct the IL2RG c. 458T>C mutation in patient T cells that carry the mutation with revertant somatic mosaicism. However, both methods failed to increase the frequency of the wild-type sequence in the mosaic T cells of patients due to limited in vitro proliferation of mutant cells and the presence of somatic reversion. To the best of our knowledge, this is the first attempt to treat mosaic cells from atypical X-SCID patients employing CRISPR/Cas9 and prime editing. We showed that prime editing can be applied to the formation of specific-point IL2RG mutations without inducing nonspecific on-target modifications. We hypothesize that the feasibility of the nucleotide substitution of the IL2RG gene using gene therapy, especially prime editing, could provide an alternative strategy to treat X-SCID patients without revertant mutations, and further technological improvements need to be developed to correct somatic mosaicism mutations.
Keywords: CRISPR/Cas9; IL2RG; SCID; prime editing; somatic mosaicism; ssODN.
Conflict of interest statement
Alicia Roig-Merino is employed at MaxCyte Inc. The authors declare no conflict of interest.
Figures
Similar articles
-
CRISPR-Cas9-AAV versus lentivector transduction for genome modification of X-linked severe combined immunodeficiency hematopoietic stem cells.Front Immunol. 2023 Jan 4;13:1067417. doi: 10.3389/fimmu.2022.1067417. eCollection 2022. Front Immunol. 2023. PMID: 36685559 Free PMC article.
-
Generation of novel Il2rg-knockout mice with clustered regularly interspaced short palindromic repeats (CRISPR) and Cas9.Exp Anim. 2020 Apr 24;69(2):189-198. doi: 10.1538/expanim.19-0120. Epub 2019 Dec 4. Exp Anim. 2020. PMID: 31801915 Free PMC article.
-
Mosaicism in CRISPR/Cas9-mediated genome editing.Dev Biol. 2019 Jan 15;445(2):156-162. doi: 10.1016/j.ydbio.2018.10.008. Epub 2018 Oct 22. Dev Biol. 2019. PMID: 30359560 Review.
-
Targeted genome editing restores T cell differentiation in a humanized X-SCID pluripotent stem cell disease model.Sci Rep. 2017 Sep 29;7(1):12475. doi: 10.1038/s41598-017-12750-4. Sci Rep. 2017. PMID: 28963568 Free PMC article.
-
CRISPR/Cas9-mediated gene editing. A promising strategy in hematological disorders.Cytotherapy. 2023 Mar;25(3):277-285. doi: 10.1016/j.jcyt.2022.11.014. Epub 2023 Jan 5. Cytotherapy. 2023. PMID: 36610813 Review.
Cited by
-
CRISPR-Cas9-Based Gene Knockout of Immune Checkpoints in Expanded NK Cells.Int J Mol Sci. 2023 Nov 8;24(22):16065. doi: 10.3390/ijms242216065. Int J Mol Sci. 2023. PMID: 38003255 Free PMC article.
-
Prime Editing for Human Gene Therapy: Where Are We Now?Cells. 2023 Feb 7;12(4):536. doi: 10.3390/cells12040536. Cells. 2023. PMID: 36831203 Free PMC article. Review.
-
Successful Correction by Prime Editing of a Mutation in the RYR1 Gene Responsible for a Myopathy.Cells. 2023 Dec 22;13(1):31. doi: 10.3390/cells13010031. Cells. 2023. PMID: 38201236 Free PMC article.
References
-
- Lim C.K., Abolhassani H., Appelberg S.K., Sundin M., Hammarström L. hypomorphic mutation: Identification of a novel pathogenic mutation in exon 8 and a review of the literature. Allergy Asthma Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol. 2019;15:2. doi: 10.1186/s13223-018-0317-y. - DOI - PMC - PubMed
-
- Stephan V., Wahn V., Le Deist F., Dirksen U., Broker B., Müller-Fleckenstein I., Horneff G., Schroten H., Fischer A., de Saint Basile G. Atypical X-linked severe combined immunodeficiency due to possible spontaneous reversion of the genetic defect in T cells. N. Engl. J. Med. 1996;335:1563–1567. doi: 10.1056/NEJM199611213352104. - DOI - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

