Investigation of the Structure and Allergic Potential of Whey Protein by Both Heating Sterilization and Simulation with Molecular Dynamics
- PMID: 36553793
- PMCID: PMC9778632
- DOI: 10.3390/foods11244050
Investigation of the Structure and Allergic Potential of Whey Protein by Both Heating Sterilization and Simulation with Molecular Dynamics
Abstract
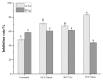
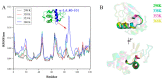
As the main allergens in milk, whey proteins are heat-sensitive proteins and are widespread in dairy products and items in which milk proteins are involved as food additives. The present work sought to investigate the effect of heating sterilization on the allergenicity of α-lactalbumin (α-LA) and β-lactoglobulin (β-LG), the main composite and allergen in whey protein isolate (WPI), by combining molecular dynamics with experimental techniques for detecting the spatial structure and IgE binding capacity. The structure of WPI was basically destroyed at heat sterilization conditions of 95 °C for 5 min and 65 °C for 30 min by SDS-PAGE analysis and spectroscopic analysis. In addition, α-lactalbumin (α-LA) may be more sensitive to temperature, resulting in exposure to allergic epitopes and increasing the allergic potential, while the binding capacity of β-lactoglobulin (β-LG) to IgE was reduced under 65 °C for 30 min. By the radius of gyration (Rg) and root-mean-square deviation (RMSD) plots calculated in molecular dynamics simulations, α-LA was less structurally stable at 368 K, while β-LG remained stable at higher temperatures, indicating that α-LA was more thermally sensitive. In addition, we observed that the regions significantly affected by temperatures were associated with the capacity of allergic epitopes (α-LA 80-101 and β-LG 82-93, 105-121) to bind IgE through root-mean-standard fluctuation (RMSF) plots, which may influence the two major allergens. We inferred that these regions are susceptible to structural changes after sterilization, thus affecting the allergenicity of allergens.
Keywords: heat sterilization; molecular dynamics simulation; structure and allergy; whey protein isolate.
Conflict of interest statement
All authors have no conflict of interest to declare.
Figures






Similar articles
-
Glutamine and lysine as common residues from epitopes on α-lactalbumin and β-lactoglobulin from cow milk identified by phage display technology.J Dairy Sci. 2023 Nov;106(11):7382-7395. doi: 10.3168/jds.2022-23151. Epub 2023 Aug 23. J Dairy Sci. 2023. PMID: 37641259
-
Chymotrypsin selectively digests β-lactoglobulin in whey protein isolate away from enzyme optimal conditions: potential for native α-lactalbumin purification.J Dairy Res. 2013 Feb;80(1):14-20. doi: 10.1017/S0022029912000416. J Dairy Res. 2013. PMID: 23317562
-
Comparison in structure, physicochemical and emulsifying properties of alpha lactoglobulin and beta lactalbumin exposed to prior γ-oryzanol by the multi-spectroscopic and silico methods.Int J Biol Macromol. 2024 Dec;282(Pt 2):136771. doi: 10.1016/j.ijbiomac.2024.136771. Epub 2024 Oct 21. Int J Biol Macromol. 2024. PMID: 39442849
-
Whey protein concentrates and isolates: processing and functional properties.Crit Rev Food Sci Nutr. 1993;33(6):431-76. doi: 10.1080/10408399309527643. Crit Rev Food Sci Nutr. 1993. PMID: 8216810 Review.
-
Recent perspective on cow's milk allergy and dairy nutrition.Crit Rev Food Sci Nutr. 2022;62(27):7503-7517. doi: 10.1080/10408398.2021.1915241. Epub 2021 May 13. Crit Rev Food Sci Nutr. 2022. PMID: 33983082 Review.
Cited by
-
Textural Restoration of Broiler Breast Fillets with Spaghetti Meat Myopathy, Using Two Alginate Gels Systems.Gels. 2023 Dec 21;10(1):7. doi: 10.3390/gels10010007. Gels. 2023. PMID: 38275847 Free PMC article.
-
Structural and Stability Analysis of GRP Family Allergens Pru p 7 and Cry j 7, Which Cause Pollen and Food Allergy Syndrome.Biomolecules. 2025 Feb 6;15(2):232. doi: 10.3390/biom15020232. Biomolecules. 2025. PMID: 40001535 Free PMC article.
-
Conformational Flexibility of a Lipocalin Allergen (Mus m 1): Implications for Molecular Allergy Diagnostics.Curr Issues Mol Biol. 2025 Mar 27;47(4):234. doi: 10.3390/cimb47040234. Curr Issues Mol Biol. 2025. PMID: 40699633 Free PMC article.
-
Recent Advances in Polymer-Based Biosensors for Food Safety Detection.Polymers (Basel). 2023 Jul 30;15(15):3253. doi: 10.3390/polym15153253. Polymers (Basel). 2023. PMID: 37571147 Free PMC article. Review.
-
Allergenicity of Alternative Proteins: Reduction Mechanisms and Processing Strategies.J Agric Food Chem. 2025 Apr 2;73(13):7522-7546. doi: 10.1021/acs.jafc.5c00948. Epub 2025 Mar 19. J Agric Food Chem. 2025. PMID: 40105205 Free PMC article. Review.
References
-
- Guptill A. Nature’s Perfect Food: How Milk Became America’s Drink. Rural Sociol. 2003;68:305.
-
- Costa J., Fernandes T.J., Villa C., Oliveira M.B.P.P., Mafra I. Advances in Food Allergen Analysis. John Wiley & Sons, Inc.; New York, NY, USA: 2017. Food Safety. Innovative Analytical Tools for Safety Assessment; pp. 305–360.
-
- Kaminogawa S., Totsuka M. Advanced Dairy Chemistry—1 Proteins. Springer; Boston, MA, USA: 2003. Allergenicity of milk proteins; pp. 647–674.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

