Association of Vitamin C Treatment with Clinical Outcomes for COVID-19 Patients: A Systematic Review and Meta-Analysis
- PMID: 36553979
- PMCID: PMC9777834
- DOI: 10.3390/healthcare10122456
Association of Vitamin C Treatment with Clinical Outcomes for COVID-19 Patients: A Systematic Review and Meta-Analysis
Abstract
Background: Vitamin C is an essential nutrient that serves as an antioxidant and is known to reduce the inflammatory response associated with pneumonia and acute respiratory distress syndrome in patients with the coronavirus disease (COVID-19), but its clinical effects remain controversial. Methods: This study aimed to investigate the therapeutic effect of vitamin C administration on the clinical outcomes of COVID-19 patients through a systematic review and meta-analysis. Results: Nineteen studies were selected, of which 949 participants administered vitamin C were in the intervention group, and 1816 participants were in the control group. All-cause mortality, hospitalization duration, length of intensive care unit stay, and ventilation incidence in COVID-19 patients were analyzed. The intervention group tends to have a lower risk ratio (RR = 0.81, 95% CI: 0.62 to 1.07; I2 = 58%; Q = 40.95; p < 0.01) in all-cause mortality than the control group. However, there were no significant differences in ventilation incidence, hospitalization duration, and length of ICU stay between the two groups. In the subgroup analysis for all-cause mortality, the risk ratio for RCT as study design, combination therapy, of vitamin C was lower than that of the combination therapy with other agents. A moderate dosage showed a lower RR than a higher dose. Conclusion: The results suggest that vitamin C may lower mortality in COVID-19 patients, but further large-scale studies are required to assess the role of vitamin C in the treatment of COVID-19.
Keywords: COVID-19; hospitalization; meta-analysis; mortality; systematic review; vitamin C.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
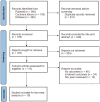
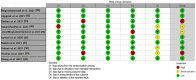
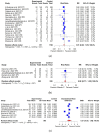




References
-
- World Health Organization Coronavirus (COVID-19) Dashboard. [(accessed on 30 May 2022)]. Available online: https://covid19.who.int/
-
- Ghinai I., McPherson T.D., Hunter J.C., Kirking H.L., Christiansen D., Joshi K., Rubin R., Morales-Estrada S., Black S.R., Pacilli M., et al. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–1144. doi: 10.1016/S0140-6736(20)30607-3. - DOI - PMC - PubMed
-
- Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C.Y., Poon R.W.S., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

