Regular Exercise Rescues Heart Function Defects and Shortens the Lifespan of Drosophila Caused by dMnM Downregulation
- PMID: 36554435
- PMCID: PMC9779684
- DOI: 10.3390/ijerph192416554
Regular Exercise Rescues Heart Function Defects and Shortens the Lifespan of Drosophila Caused by dMnM Downregulation
Abstract
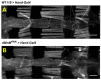
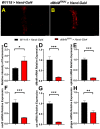
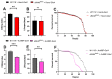
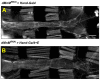
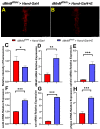
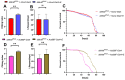
Although studies have shown that myomesin 2 (MYOM2) mutations can lead to hypertrophic cardiomyopathy (HCM), a common cardiovascular disease that has a serious impact on human life, the effect of MYOM2 on cardiac function and lifespan in humans is unknown. In this study, dMnM (MYOM2 homologs) knockdown in cardiomyocytes resulted in diastolic cardiac defects (diastolic dysfunction and arrhythmias) and increased cardiac oxidative stress. Furthermore, the knockdown of dMnM in indirect flight muscle (IFM) reduced climbing ability and shortened lifespan. However, regular exercise significantly ameliorated diastolic cardiac dysfunction, arrhythmias, and oxidative stress triggered by dMnM knockdown in cardiac myocytes and also reversed the reduction in climbing ability and shortening of lifespan caused by dMnM knockdown in Drosophila IFM. In conclusion, these results suggest that Drosophila cardiomyocyte dMnM knockdown leads to cardiac functional defects, while dMnM knockdown in IFM affects climbing ability and lifespan. Furthermore, regular exercise effectively upregulates cardiomyocyte dMnM expression levels and ameliorates cardiac functional defects caused by Drosophila cardiomyocyte dMnM knockdown by increasing cardiac antioxidant capacity. Importantly, regular exercise ameliorates the shortened lifespan caused by dMnM knockdown in IFM.
Keywords: IFM; cardiac function; dMnM; lifespan; regular exercise.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

