Epigenomic and Other Evidence for Cannabis-Induced Aging Contextualized in a Synthetic Epidemiologic Overview of Cannabinoid-Related Teratogenesis and Cannabinoid-Related Carcinogenesis
- PMID: 36554603
- PMCID: PMC9778714
- DOI: 10.3390/ijerph192416721
Epigenomic and Other Evidence for Cannabis-Induced Aging Contextualized in a Synthetic Epidemiologic Overview of Cannabinoid-Related Teratogenesis and Cannabinoid-Related Carcinogenesis
Abstract
Background: Twelve separate streams of empirical data make a strong case for cannabis-induced accelerated aging including hormonal, mitochondriopathic, cardiovascular, hepatotoxic, immunological, genotoxic, epigenotoxic, disruption of chromosomal physiology, congenital anomalies, cancers including inheritable tumorigenesis, telomerase inhibition and elevated mortality.
Methods: Results from a recently published longitudinal epigenomic screen were analyzed with regard to the results of recent large epidemiological studies of the causal impacts of cannabis. We also integrate theoretical syntheses with prior studies into these combined epigenomic and epidemiological results.
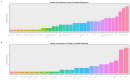
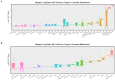
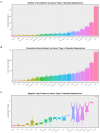
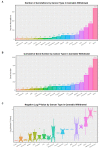
Results: Cannabis dependence not only recapitulates many of the key features of aging, but is characterized by both age-defining and age-generating illnesses including immunomodulation, hepatic inflammation, many psychiatric syndromes with a neuroinflammatory basis, genotoxicity and epigenotoxicity. DNA breaks, chromosomal breakage-fusion-bridge morphologies and likely cycles, and altered intergenerational DNA methylation and disruption of both the histone and tubulin codes in the context of increased clinical congenital anomalies, cancers and heritable tumors imply widespread disruption of the genome and epigenome. Modern epigenomic clocks indicate that, in cannabis-dependent patients, cannabis advances cellular DNA methylation age by 25-30% at age 30 years. Data have implications not only for somatic but also stem cell and germ line tissues including post-fertilization zygotes. This effect is likely increases with the square of chronological age.
Conclusion: Recent epigenomic studies of cannabis exposure provide many explanations for the broad spectrum of cannabis-related teratogenicity and carcinogenicity and appear to account for many epidemiologically observed findings. Further research is indicated on the role of cannabinoids in the aging process both developmentally and longitudinally, from stem cell to germ cell to blastocystoids to embryoid bodies and beyond.
Keywords: DNA methylation; ageing; aging; cannabis; epigenotoxicity; genotoxicity; teratology.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Salvioli S., Monti D., Lanzarini C., Conte M., Pirazzini C., Bacalini M.G., Garagnani P., Giuliani C., Fontanesi E., Ostan R., et al. Immune system, cell senescence, aging and longevity--inflamm-aging reappraised. Curr. Pharm. Des. 2013;19:1675–1679. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

