The Role of microRNAs in Inflammation
- PMID: 36555120
- PMCID: PMC9779565
- DOI: 10.3390/ijms232415479
The Role of microRNAs in Inflammation
Abstract
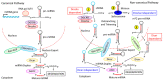
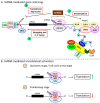
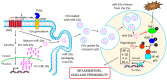
Inflammation is a biological response of the immune system to various insults, such as pathogens, toxic compounds, damaged cells, and radiation. The complex network of pro- and anti-inflammatory factors and their direction towards inflammation often leads to the development and progression of various inflammation-associated diseases. The role of small non-coding RNAs (small ncRNAs) in inflammation has gained much attention in the past two decades for their regulation of inflammatory gene expression at multiple levels and their potential to serve as biomarkers and therapeutic targets in various diseases. One group of small ncRNAs, microRNAs (miRNAs), has become a key regulator in various inflammatory disease conditions. Their fine-tuning of target gene regulation often turns out to be an important factor in controlling aberrant inflammatory reactions in the system. This review summarizes the biogenesis of miRNA and the mechanisms of miRNA-mediated gene regulation. The review also briefly discusses various pro- and anti-inflammatory miRNAs, their targets and functions, and provides a detailed discussion on the role of miR-10a in inflammation.
Keywords: inflammation; microRNA; small non-coding RNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Parke D.V., Parke A.L. Chemical-induced inflammation and inflammatory diseases. Int. J. Occup. Med. Environ. Health. 1996;9:211–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

