An Overlooked Hepcidin-Cadmium Connection
- PMID: 36555126
- PMCID: PMC9779829
- DOI: 10.3390/ijms232415483
An Overlooked Hepcidin-Cadmium Connection
Abstract
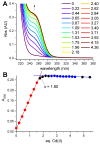
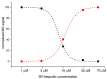
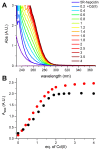
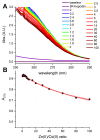
Hepcidin (DTHFPICIFCCGCCHRSKCGMCCKT), an iron-regulatory hormone, is a 25-amino-acid peptide with four intramolecular disulfide bonds circulating in blood. Its hormonal activity is indirect and consists of marking ferroportin-1 (an iron exporter) for degradation. Hepcidin biosynthesis involves the N-terminally extended precursors prepro-hepcidin and pro-hepcidin, processed by peptidases to the final 25-peptide form. A sequence-specific formation of disulfide bonds and export of the oxidized peptide to the bloodstream follows. In this study we considered the fact that prior to export, reduced hepcidin may function as an octathiol ligand bearing some resemblance to the N-terminal part of the α-domain of metallothioneins. Consequently, we studied its ability to bind Zn(II) and Cd(II) ions using the original peptide and a model for prohepcidin extended N-terminally with a stretch of five arginine residues (5R-hepcidin). We found that both form equivalent mononuclear complexes with two Zn(II) or Cd(II) ions saturating all eight Cys residues. The average affinity at pH 7.4, determined from pH-metric spectroscopic titrations, is 1010.1 M-1 for Zn(II) ions; Cd(II) ions bind with affinities of 1015.2 M-1 and 1014.1 M-1. Using mass spectrometry and 5R-hepcidin we demonstrated that hepcidin can compete for Cd(II) ions with metallothionein-2, a cellular cadmium target. This study enabled us to conclude that hepcidin binds Zn(II) and Cd(II) sufficiently strongly to participate in zinc physiology and cadmium toxicity under intracellular conditions.
Keywords: MT2A; affinity constant; cadmium; hepcidin; metallothionein; prohepcidin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Maret W. Advances in Experimental Medicine and Biology. Volume 1055. Springer; New York, NY, USA: 2018. Metallomics: The Science of Biometals and Biometalloids; pp. 1–20. - PubMed
-
- Płonka D., Bal W. The N-Terminus of Hepcidin Is a Strong and Potentially Biologically Relevant Cu(II) Chelator. Inorganica Chim. Acta. 2018;472:76–81. doi: 10.1016/j.ica.2017.06.051. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

