Impacts of Telomeric Length, Chronic Hypoxia, Senescence, and Senescence-Associated Secretory Phenotype on the Development of Thoracic Aortic Aneurysm
- PMID: 36555139
- PMCID: PMC9779024
- DOI: 10.3390/ijms232415498
Impacts of Telomeric Length, Chronic Hypoxia, Senescence, and Senescence-Associated Secretory Phenotype on the Development of Thoracic Aortic Aneurysm
Abstract
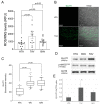
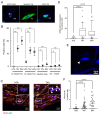
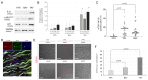
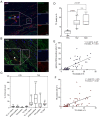
Thoracic aortic aneurysm (TAA) is an age-related and life-threatening vascular disease. Telomere shortening is a predictor of age-related diseases, and its progression is associated with premature vascular disease. The aim of the present work was to investigate the impacts of chronic hypoxia and telomeric DNA damage on cellular homeostasis and vascular degeneration of TAA. We analyzed healthy and aortic aneurysm specimens (215 samples) for telomere length (TL), chronic DNA damage, and resulting changes in cellular homeostasis, focusing on senescence and apoptosis. Compared with healthy thoracic aorta (HTA), patients with tricuspid aortic valve (TAV) showed telomere shortening with increasing TAA size, in contrast to genetically predisposed bicuspid aortic valve (BAV). In addition, TL was associated with chronic hypoxia and telomeric DNA damage and with the induction of senescence-associated secretory phenotype (SASP). TAA-TAV specimens showed a significant difference in SASP-marker expression of IL-6, NF-κB, mTOR, and cell-cycle regulators (γH2AX, Rb, p53, p21), compared to HTA and TAA-BAV. Furthermore, we observed an increase in CD163+ macrophages and a correlation between hypoxic DNA damage and the number of aortic telocytes. We conclude that chronic hypoxia is associated with telomeric DNA damage and the induction of SASP in a diseased aortic wall, promising a new therapeutic target.
Keywords: DNA damage; aneurysm; cell death; senescence-associated secretory phenotype; telocytes; telomere; thoracic aorta.
Conflict of interest statement
The authors confirm that there is no conflict of interest.
Figures

Similar articles
-
Targeted gene expression analyses and immunohistology suggest a pro-proliferative state in tricuspid aortic valve-, and senescence and viral infections in bicuspid aortic valve-associated thoracic aortic aneurysms.Atherosclerosis. 2018 Apr;271:111-119. doi: 10.1016/j.atherosclerosis.2018.02.007. Epub 2018 Feb 5. Atherosclerosis. 2018. PMID: 29486395
-
Analysis of Vascular Smooth Muscle Cells from Thoracic Aortic Aneurysms Reveals DNA Damage and Cell Cycle Arrest as Hallmarks in Bicuspid Aortic Valve Patients.J Proteome Res. 2024 Aug 2;23(8):3012-3024. doi: 10.1021/acs.jproteome.3c00649. Epub 2024 Apr 9. J Proteome Res. 2024. PMID: 38594816 Free PMC article.
-
Focus on the unique mechanisms involved in thoracic aortic aneurysm formation in bicuspid aortic valve versus tricuspid aortic valve patients: clinical implications of a pilot study.Eur J Cardiothorac Surg. 2013 Jun;43(6):e180-6. doi: 10.1093/ejcts/ezs630. Epub 2012 Dec 17. Eur J Cardiothorac Surg. 2013. PMID: 23248206
-
Differential expression of MMP-2, MMP-9 and TIMP proteins in thoracic aortic aneurysm - comparison with and without bicuspid aortic valve: a meta-analysis.Vasa. 2014 Nov;43(6):433-42. doi: 10.1024/0301-1526/a000390. Vasa. 2014. PMID: 25339161 Review.
-
Is there a role for autophagy in ascending aortopathy associated with tricuspid or bicuspid aortic valve?Clin Sci (Lond). 2019 Apr 2;133(7):805-819. doi: 10.1042/CS20181092. Print 2019 Apr 15. Clin Sci (Lond). 2019. PMID: 30991346 Review.
Cited by
-
SenNet recommendations for detecting senescent cells in different tissues.Nat Rev Mol Cell Biol. 2024 Dec;25(12):1001-1023. doi: 10.1038/s41580-024-00738-8. Epub 2024 Jun 3. Nat Rev Mol Cell Biol. 2024. PMID: 38831121 Review.
-
Increased vascular smooth muscle cell senescence in aneurysmal Fibulin-4 mutant mice.NPJ Aging. 2024 Jun 20;10(1):31. doi: 10.1038/s41514-024-00154-4. NPJ Aging. 2024. PMID: 38902222 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

