Primary Cilia Restrain PI3K-AKT Signaling to Orchestrate Human Decidualization
- PMID: 36555215
- PMCID: PMC9779442
- DOI: 10.3390/ijms232415573
Primary Cilia Restrain PI3K-AKT Signaling to Orchestrate Human Decidualization
Abstract
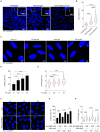
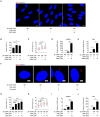
Endometrial decidualization plays a pivotal role during early pregnancy. Compromised decidualization has been tightly associated with recurrent implantation failure (RIF). Primary cilium is an antenna-like sensory organelle and acts as a signaling nexus to mediate Hh, Wnt, TGFβ, BMP, FGF, and Notch signaling. However, whether primary cilium is involved in human decidualization is still unknown. In this study, we found that primary cilia are present in human endometrial stromal cells. The ciliogenesis and cilia length are increased by progesterone during in vitro and in vivo decidualization. Primary cilia are abnormal in the endometrium of RIF patients. Based on data from both assembly and disassembly of primary cilia, it has been determined that primary cilium is essential to human decidualization. Trichoplein (TCHP)-Aurora A signaling mediates cilia disassembly during human in vitro decidualization. Mechanistically, primary cilium modulates human decidualization through PTEN-PI3K-AKT-FOXO1 signaling. Our study highlights primary cilium as a novel decidualization-related signaling pathway.
Keywords: AKT; Aurora A; decidualization; primary cilium.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

