Hydroxychloroquine Mitigates Dilated Cardiomyopathy Phenotype in Transgenic D94A Mice
- PMID: 36555229
- PMCID: PMC9779604
- DOI: 10.3390/ijms232415589
Hydroxychloroquine Mitigates Dilated Cardiomyopathy Phenotype in Transgenic D94A Mice
Abstract
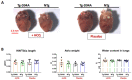
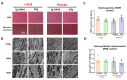
In this study, we aimed to investigate whether short-term and low-dose treatment with hydroxychloroquine (HCQ), an antimalarial drug, can modulate heart function in a preclinical model of dilated cardiomyopathy (DCM) expressing the D94A mutation in cardiac myosin regulatory light chain (RLC) compared with healthy non-transgenic (NTg) littermates. Increased interest in HCQ came with the COVID-19 pandemic, but the risk of cardiotoxic side effects of HCQ raised concerns, especially in patients with an underlying heart condition, e.g., cardiomyopathy. Effects of HCQ treatment vs. placebo (H2O), administered in Tg-D94A vs. NTg mice over one month, were studied by echocardiography and muscle contractile mechanics. Global longitudinal strain analysis showed the HCQ-mediated improvement in heart performance in DCM mice. At the molecular level, HCQ promoted the switch from myosin's super-relaxed (SRX) to disordered relaxed (DRX) state in DCM-D94A hearts. This result indicated more myosin cross-bridges exiting a hypocontractile SRX-OFF state and assuming the DRX-ON state, thus potentially enhancing myosin motor function in DCM mice. This bottom-up investigation of the pharmacological use of HCQ at the level of myosin molecules, muscle fibers, and whole hearts provides novel insights into mechanisms by which HCQ therapy mitigates some abnormal phenotypes in DCM-D94A mice and causes no harm in healthy NTg hearts.
Keywords: MYL2 gene; dilated cardiomyopathy (DCM); echocardiography; hydroxychloroquine (HCQ); regulatory light chain (RLC); super-relaxed state; transgenic mice.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Wang N., Han S., Liu R., Meng L., He H., Zhang Y., Wang C., Lv Y., Wang J., Li X., et al. Chloroquine and hydroxychloroquine as ACE2 blockers to inhibit viropexis of 2019-nCoV Spike pseudotyped virus. Phytomedicine Int. J. Phytother. PhytoPharm. 2020;79:153333. doi: 10.1016/j.phymed.2020.153333. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

