SP600125 Enhances Temperature-Controlled Repeated Thermal Stimulation-Induced Neurite Outgrowth in PC12-P1F1 Cells
- PMID: 36555248
- PMCID: PMC9779509
- DOI: 10.3390/ijms232415602
SP600125 Enhances Temperature-Controlled Repeated Thermal Stimulation-Induced Neurite Outgrowth in PC12-P1F1 Cells
Abstract
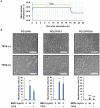
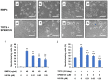
This study evaluated the mechanism of temperature-controlled repeated thermal stimulation (TRTS)-mediated neuronal differentiation. We assessed the effect of SP600125, a c-Jun N-terminal kinase (JNK) inhibitor, on neuronal differentiation of rat PC12-P1F1 cells, which can differentiate into neuron-like cells by exposure to TRTS or neurotrophic factors, including bone morphogenetic protein (BMP) 4. We evaluated neuritogenesis by incubating the cells under conditions of TRTS and/or SP600125. Cotreatment with SP600125 significantly enhanced TRTS-mediated neuritogenesis, whereas that with other selective mitogen-activated protein kinase (MAPK) inhibitors did not-e.g., extracellular signal-regulated kinase (ERK)1/2 inhibitor U0126, and p38 MAPK inhibitor SB203580. We tried to clarify the mechanism of SP600125 action by testing the effect of U0126 and the BMP receptor inhibitor LDN193189 on the SP600125-mediated enhancement of intracellular signaling. SP600125-enhanced TRTS-induced neuritogenesis was significantly inhibited by U0126 or LDN193189. Gene expression analysis revealed that TRTS significantly increased β3-Tubulin, MKK3, and Smad7 gene expressions. Additionally, Smad6 and Smad7 gene expressions were substantially attenuated through SP600125 co-treatment during TRTS. Therefore, SP600125 may partly enhance TRTS-induced neuritogenesis by attenuating the negative feedback loop of BMP signaling. Further investigation of the mechanisms underlying the effect of SP600125 during TRTS-mediated neuritogenesis may contribute to the future development of regenerative neuromedicine.
Keywords: PC12 cells; SP600125; neuritogenesis; temperature-controlled repeated thermal stimulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Teng K.K., Georgieff I.S., Nunez J., Shelanski M.L., Greene L.A. Characterization of a PC12 cell sub-clone (PC12-C41) with enhanced neurite outgrowth with capacity: Implications for a modulatory role of high molecular weight tau in neuritogenesis. J. Cell Sci. 1993;106:611–626. doi: 10.1242/jcs.106.2.611. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

