Magnetic Luffa-Leaf-Derived Hierarchical Porous Biochar for Efficient Removal of Rhodamine B and Tetracycline Hydrochloride
- PMID: 36555345
- PMCID: PMC9779706
- DOI: 10.3390/ijms232415703
Magnetic Luffa-Leaf-Derived Hierarchical Porous Biochar for Efficient Removal of Rhodamine B and Tetracycline Hydrochloride
Abstract
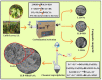
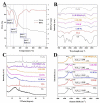
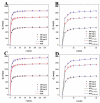
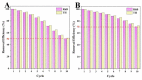
Luffa leaf (LL) is an agricultural waste produced by loofah. In this work, LL was used as biomass carbon source for biochars for the first time. After carbonization, activation, and chemical co-precipitation treatments, a magnetic lignocellulose-derived hierarchical porous biochar was obtained. The specific surface area and total pore volume were 2565.4 m2/g and 1.4643 cm3/g, and the surface was rich in carbon and oxygen functional groups. The synthetic dye rhodamine B (RhB) and the antibiotic tetracycline hydrochloride (TH) were selected as organic pollutant models to explore the ability to remove organic pollutants, and the results showed good adsorption performances. The maximum adsorption capacities were 1701.7 mg/g for RhB and 1755.9 mg/g for TH, which were higher than most carbon-based adsorbents. After 10 cycles of use, the removal efficiencies were still maintained at more than 70%, showing good stability. This work not only verified the feasibility of lignocellulose LL as a carbon source to prepare biochar but also prepared a magnetic hierarchical porous adsorbent with good performances that can better treat RhB and TH, which provided a new idea and direction for the efficient removal of organic pollutants in water.
Keywords: efficient removal; hierarchical porous; lignocellulose; magnetic biochar; organic pollutants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Dapaah M.F., Niu Q., Yu Y., You T., Liu B., Cheng L. Efficient persistent organic pollutant removal in water using MIL-metal–organic framework driven Fenton-like reactions: A critical review. Chem. Eng. J. 2022;431:134182. doi: 10.1016/j.cej.2021.134182. - DOI
-
- Christensen E.R., Wang Y., Huo J., Li A. Properties and fate and transport of persistent and mobile polar organic water pollutants: A review. J. Environ. Chem. Eng. 2022;10:107201. doi: 10.1016/j.jece.2022.107201. - DOI
-
- Naghdi S., Shahrestani M.M., Zendehbad M., Djahaniani H., Kazemian H., Eder D. Recent advances in application of metal-organic frameworks (MOFs) as adsorbent and catalyst in removal of persistent organic pollutants (POPs) J. Hazard. Mater. 2023;442:130127. doi: 10.1016/j.jhazmat.2022.130127. - DOI - PubMed
-
- Du C., Zhang Y., Zhang Z., Zhou L., Yu G., Wen X., Chi T., Wang G., Su Y., Deng F., et al. Fe-based metal organic frameworks (Fe-MOFs) for organic pollutants removal via photo-Fenton: A review. Chem. Eng. J. 2022;431:133932. doi: 10.1016/j.cej.2021.133932. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

