The J Domain of Sacsin Disrupts Intermediate Filament Assembly
- PMID: 36555380
- PMCID: PMC9779362
- DOI: 10.3390/ijms232415742
The J Domain of Sacsin Disrupts Intermediate Filament Assembly
Abstract
Autosomal Recessive Spastic Ataxia of the Charlevoix Saguenay (ARSACS) is caused by mutation in the SACS gene resulting in loss of function of the protein sacsin. A key feature is the formation of abnormal bundles of neurofilaments (NF) in neurons and vimentin intermediate filaments (IF) in cultured fibroblasts, suggesting a role of sacsin in IF homeostasis. Sacsin contains a J domain (SacsJ) homologous to Hsp40, that can interact with Hsp70 chaperones. The SacsJ domain resolved NF bundles in cultured Sacs-/- neurons. Having studied the mechanism using NF assembled in vitro from purified NF proteins, we report that the SacsJ domain interacts with NF proteins to disassemble NFL filaments, and to inhibit their initial assembly. A cell-penetrating peptide derived from this domain, SacsJ-myc-TAT was efficient in disassembling NF bundles in cultured Sacs-/- motor neurons, restoring the NF network; however, there was some loss of vimentin IF and NF in cultured Sacs+/+ fibroblasts and motor neurons, respectively. These results suggest that sacsin through its SacsJ domain is a key regulator of NF and vimentin IF networks in cells.
Keywords: J domain; ataxia; chaperone; intermediate filaments; motor neuron; neurofilament; vimentin.
Conflict of interest statement
The authors declare no conflict of interest. The authors declare no competing financial interest.
Figures
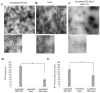
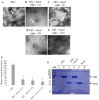

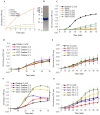
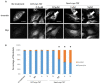
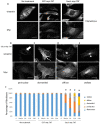
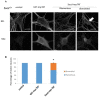


References
-
- Vingolo E.M., Di Fabio R., Salvatore S., Grieco G., Bertini E., Leuzzi V., Nesti C., Filla A., Tessa A., Pierelli F., et al. Myelinated retinal fibers in autosomal recessive spastic ataxia of Charlevoix-Saguenay. Eur. J. Neurol. 2011;18:1187–1190. doi: 10.1111/j.1468-1331.2010.03335.x. - DOI - PubMed
-
- Girard M., Larivière R., Parfitt D.A., Deane E.C., Gaudet R., Nossova N., Blondeau F., Prenosil G., Vermeulen E.G.M., Duchen M.R., et al. Mitochondrial dysfunction and Purkinje cell loss in autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) Proc. Natl. Acad. Sci. USA. 2012;109:1661–1666. doi: 10.1073/pnas.1113166109. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

