Olfactory Gene Families in Scopula subpunctaria and Candidates for Type-II Sex Pheromone Detection
- PMID: 36555416
- PMCID: PMC9779464
- DOI: 10.3390/ijms232415775
Olfactory Gene Families in Scopula subpunctaria and Candidates for Type-II Sex Pheromone Detection
Abstract
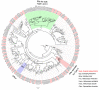
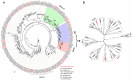
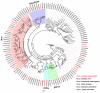
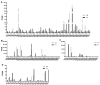
Scopula subpunctaria, an abundant pest in tea gardens, produce type-II sex pheromone components, which are critical for its communicative and reproductive abilities; however, genes encoding the proteins involved in the detection of type-II sex pheromone components have rarely been documented in moths. In the present study, we sequenced the transcriptomes of the male and female S. subpunctaria antennae. A total of 150 candidate olfaction genes, comprising 58 odorant receptors (SsubORs), 26 ionotropic receptors (SsubIRs), 24 chemosensory proteins (SsubCSPs), 40 odorant-binding proteins (SsubOBPs), and 2 sensory neuron membrane proteins (SsubSNMPs) were identified in S. subpunctaria. Phylogenetic analysis, qPCR, and mRNA abundance analysis results suggested that SsubOR46 may be the Orco (non-traditional odorant receptor, a subfamily of ORs) of S. subpunctaria. SsubOR9, SsubOR53, and SsubOR55 belonged to the pheromone receptor (PR) clades which have a higher expression in male antennae. Interestingly, SsubOR44 was uniquely expressed in the antennae, with a higher expression in males than in females. SsubOBP25, SsubOBP27, and SsubOBP28 were clustered into the moth pheromone-binding protein (PBP) sub-family, and they were uniquely expressed in the antennae, with a higher expression in males than in females. SsubOBP19, a member of the GOBP2 group, was the most abundant OBP in the antennae. These findings indicate that these olfactory genes, comprising five candidate PRs, three candidate PBPs, and one candidate GOBP2, may be involved in type II sex pheromone detection. As well as these genes, most of the remaining SsubORs, and all of the SsubIRs, showed a considerably higher expression in the female antennae than in the male antennae. Many of these, including SsubOR40, SsubOR42, SsubOR43, and SsubIR26, were more abundant in female antennae. These olfactory and ionotropic receptors may be related to the detection of host plant volatiles. The results of this present study provide a basis for exploring the olfaction mechanisms in S. subpunctaria, with a focus on the genes involved in type II sex pheromones. The evolutionary analyses in our study provide new insights into the differentiation and evolution of lepidopteran PRs.
Keywords: Scopula subpunctaria; olfactory gene; sex pheromone perception; transcriptomic analysis; type-II sex pheromone.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Transcriptome Profiling of Euproctis pseudoconspersa Reveals Candidate Olfactory Genes for Type III Sex Pheromone Detection.Int J Mol Sci. 2025 Feb 7;26(4):1405. doi: 10.3390/ijms26041405. Int J Mol Sci. 2025. PMID: 40003873 Free PMC article.
-
Identification of cytochrome P450, odorant-binding protein, and chemosensory protein genes involved in Type II sex pheromone biosynthesis and transportation in the tea pest, Scopula subpunctaria.Pestic Biochem Physiol. 2020 Oct;169:104650. doi: 10.1016/j.pestbp.2020.104650. Epub 2020 Jul 9. Pestic Biochem Physiol. 2020. PMID: 32828368
-
Antennal transcriptome analysis of the piercing moth Oraesia emarginata (Lepidoptera: Noctuidae).PLoS One. 2017 Jun 14;12(6):e0179433. doi: 10.1371/journal.pone.0179433. eCollection 2017. PLoS One. 2017. PMID: 28614384 Free PMC article.
-
Antennal transcriptome analysis and comparison of olfactory genes in two sympatric defoliators, Dendrolimus houi and Dendrolimus kikuchii (Lepidoptera: Lasiocampidae).Insect Biochem Mol Biol. 2014 Sep;52:69-81. doi: 10.1016/j.ibmb.2014.06.006. Epub 2014 Jul 3. Insect Biochem Mol Biol. 2014. PMID: 24998398
-
Olfactory perireceptor and receptor events in moths: a kinetic model revised.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009 Oct;195(10):895-922. doi: 10.1007/s00359-009-0461-4. Epub 2009 Aug 21. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009. PMID: 19697043 Free PMC article. Review.
Cited by
-
Transcriptome Profiling of Euproctis pseudoconspersa Reveals Candidate Olfactory Genes for Type III Sex Pheromone Detection.Int J Mol Sci. 2025 Feb 7;26(4):1405. doi: 10.3390/ijms26041405. Int J Mol Sci. 2025. PMID: 40003873 Free PMC article.
-
Identification of Candidate Genes Associated with Type-II Sex Pheromone Biosynthesis in the Tea Geometrid (Ectropis obliqua) (Lepidoptera: Geometridae).Insects. 2024 Apr 15;15(4):276. doi: 10.3390/insects15040276. Insects. 2024. PMID: 38667406 Free PMC article.
References
-
- Segura-Leon O.L., Torres-Huerta B., Estrada-Perez A.R., Cibrian-Tovar J., Hernandez-Hernandez F.C., Cruz-Jaramillo J.L., Meza-Hernandez J.S., Sanchez-Galicia F. Identification of candidate chemosensory gene families by head transcriptomes analysis in the mexican fruit fly, Anastrepha ludens Loew (Diptera: Tephritidae) Int. J. Mol. Sci. 2022;23:10531. doi: 10.3390/ijms231810531. - DOI - PMC - PubMed
-
- Liu J., Li Z.Q., Luo Z.X., Cai X.M., Bian L., Xin Z.J., Chen Z.M. Comparison of male antennal morphology and sensilla physiology for sex pheromone olfactory sensing between sibling moth species: Ectropis grisescens and Ectropis obliqua (Geometridae) Arch. Insect Biochem. Physiol. 2019;101:e21545. doi: 10.1002/arch.21545. - DOI - PubMed
-
- Lofstedt C., Wahlberg N., Millar J.G. Evolutionary Patterns of Pheromone Diversity in Lepidoptera. University of California Press; Oakland, CA, USA: 2016. pp. 43–78.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

