Neural Stem Cells Overexpressing Arginine Decarboxylase Improve Functional Recovery from Spinal Cord Injury in a Mouse Model
- PMID: 36555425
- PMCID: PMC9779865
- DOI: 10.3390/ijms232415784
Neural Stem Cells Overexpressing Arginine Decarboxylase Improve Functional Recovery from Spinal Cord Injury in a Mouse Model
Abstract
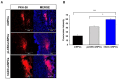
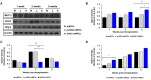
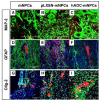
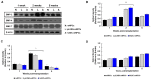
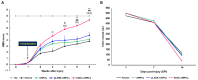
Current therapeutic strategies for spinal cord injury (SCI) cannot fully facilitate neural regeneration or improve function. Arginine decarboxylase (ADC) synthesizes agmatine, an endogenous primary amine with neuroprotective effects. Transfection of human ADC (hADC) gene exerts protective effects after injury in murine brain-derived neural precursor cells (mNPCs). Following from these findings, we investigated the effects of hADC-mNPC transplantation in SCI model mice. Mice with experimentally damaged spinal cords were divided into three groups, separately transplanted with fluorescently labeled (1) control mNPCs, (2) retroviral vector (pLXSN)-infected mNPCs (pLXSN-mNPCs), and (3) hADC-mNPCs. Behavioral comparisons between groups were conducted weekly up to 6 weeks after SCI, and urine volume was measured up to 2 weeks after SCI. A subset of animals was euthanized each week after cell transplantation for molecular and histological analyses. The transplantation groups experienced significantly improved behavioral function, with the best recovery occurring in hADC-mNPC mice. Transplanting hADC-mNPCs improved neurological outcomes, induced oligodendrocyte differentiation and remyelination, increased neural lineage differentiation, and decreased glial scar formation. Moreover, locomotor and bladder function were both rehabilitated. These beneficial effects are likely related to differential BMP-2/4/7 expression in neuronal cells, providing an empirical basis for gene therapy as a curative SCI treatment option.
Keywords: agmatine; arginine decarboxylase; axonal re-myelination; cell transplantation; functional recovery; gene therapy; glial scar; neural progenitor cells; neurogenesis; spinal cord injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Restorative benefits of transplanting human mesenchymal stromal cells overexpressing arginine decarboxylase genes after spinal cord injury.Cytotherapy. 2015 Jan;17(1):25-37. doi: 10.1016/j.jcyt.2014.08.006. Epub 2014 Oct 22. Cytotherapy. 2015. PMID: 25442787
-
Transfection of arginine decarboxylase gene increases the neuronal differentiation of neural progenitor cells.Stem Cell Res. 2016 Sep;17(2):256-265. doi: 10.1016/j.scr.2016.08.009. Epub 2016 Aug 17. Stem Cell Res. 2016. PMID: 27591482
-
The multifaceted effects of agmatine on functional recovery after spinal cord injury through Modulations of BMP-2/4/7 expressions in neurons and glial cells.PLoS One. 2013;8(1):e53911. doi: 10.1371/journal.pone.0053911. Epub 2013 Jan 21. PLoS One. 2013. PMID: 23349763 Free PMC article.
-
Direct neuronal differentiation of neural stem cells for spinal cord injury repair.Stem Cells. 2021 Aug;39(8):1025-1032. doi: 10.1002/stem.3366. Epub 2021 Mar 5. Stem Cells. 2021. PMID: 33657255 Review.
-
Stem cell-based cell therapy for spinal cord injury.Cell Transplant. 2007;16(4):355-64. doi: 10.3727/000000007783464885. Cell Transplant. 2007. PMID: 17658126 Review.
Cited by
-
Advances in genetically modified neural stem cell therapy for central nervous system injury and neurological diseases.Stem Cell Res Ther. 2024 Dec 18;15(1):482. doi: 10.1186/s13287-024-04089-1. Stem Cell Res Ther. 2024. PMID: 39696712 Free PMC article. Review.
-
Oligodendrocyte Progenitors in Glial Scar: A Bet on Remyelination.Cells. 2024 Jun 12;13(12):1024. doi: 10.3390/cells13121024. Cells. 2024. PMID: 38920654 Free PMC article. Review.
-
Special Issue "Stem Cell Biology & Regenerative Medicine".Int J Mol Sci. 2023 Aug 16;24(16):12855. doi: 10.3390/ijms241612855. Int J Mol Sci. 2023. PMID: 37629035 Free PMC article.
-
Possible Role of Cellular Polyamine Metabolism in Neuronal Apoptosis.Curr Med Sci. 2024 Apr;44(2):281-290. doi: 10.1007/s11596-024-2843-9. Epub 2024 Mar 8. Curr Med Sci. 2024. PMID: 38453792
References
-
- Oyinbo C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. (Wars) 2011;71:281–299. - PubMed
-
- Anjum A., Yazid M.D., Fauzi Daud M., Idris J., Ng A.M.H., Selvi Naicker A., Ismail O.H.R., Athi Kumar R.K., Lokanathan Y. Spinal cord injury: Pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int. J. Mol. Sci. 2020;21:7533. doi: 10.3390/ijms21207533. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

