Semiclassical Theory of Multistage Nonequilibrium Electron Transfer in Macromolecular Compounds in Polar Media with Several Relaxation Timescales
- PMID: 36555434
- PMCID: PMC9779366
- DOI: 10.3390/ijms232415793
Semiclassical Theory of Multistage Nonequilibrium Electron Transfer in Macromolecular Compounds in Polar Media with Several Relaxation Timescales
Abstract
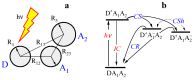
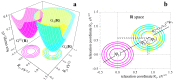
Many specific features of ultrafast electron transfer (ET) reactions in macromolecular compounds can be attributed to nonequilibrium configurations of intramolecular vibrational degrees of freedom and the environment. In photoinduced ET, nonequilibrium nuclear configurations are often produced at the stage of optical excitation, but they can also be the result of electron tunneling itself, i.e., fast redistribution of charges within the macromolecule. A consistent theoretical description of ultrafast ET requires an explicit consideration of the nuclear subsystem, including its evolution between electron jumps. In this paper, the effect of the multi-timescale nuclear reorganization on ET transitions in macromolecular compounds is studied, and a general theory of ultrafast ET in non-Debye polar environments with a multi-component relaxation function is developed. Particular attention is paid to designing the multidimensional space of nonequilibrium nuclear configurations, as well as constructing the diabatic free energy surfaces for the ET states. The reorganization energies of individual ET transitions, the equilibrium energies of ET states, and the relaxation properties of the environment are used as input data for the theory. The effect of the system-environment interaction on the ET kinetics is discussed, and mechanisms for enhancing the efficiency of charge separation in macromolecular compounds are analyzed.
Keywords: electron transfer; macromolecular compounds; nonequilibrium processes; photochemistry; ultrafast reactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Solvent-assisted multistage nonequilibrium electron transfer in rigid supramolecular systems: Diabatic free energy surfaces and algorithms for numerical simulations.J Chem Phys. 2018 Mar 14;148(10):104107. doi: 10.1063/1.5016438. J Chem Phys. 2018. PMID: 29544284
-
Multidimensional treatment of stochastic solvent dynamics in photoinduced proton-coupled electron transfer processes: sequential, concerted, and complex branching mechanisms.J Chem Phys. 2011 Oct 14;135(14):144115. doi: 10.1063/1.3651083. J Chem Phys. 2011. PMID: 22010706
-
Ultrafast nonequilibrium dynamics of short-range protein electron transfer in flavodoxin.Phys Chem Chem Phys. 2021 Dec 22;24(1):382-391. doi: 10.1039/d1cp04445a. Phys Chem Chem Phys. 2021. PMID: 34889914
-
Charge transfer in dynamical biosystems, or the treachery of (static) images.Acc Chem Res. 2015 Feb 17;48(2):474-81. doi: 10.1021/ar500271d. Epub 2014 Oct 13. Acc Chem Res. 2015. PMID: 25307316 Free PMC article. Review.
-
Reorganization energy of electron transfer.Phys Chem Chem Phys. 2023 Mar 15;25(11):7589-7610. doi: 10.1039/d2cp06072h. Phys Chem Chem Phys. 2023. PMID: 36876860 Review.
References
-
- Williams R.J.P. Electron Transfer in Biology and the Solid State. American Chemical Society; Washington, DC, USA: 1990. Overview of Biological Electron Transfer; pp. 3–23. Chapter 1. - DOI
-
- Formosinho S., Barroso M., editors. Proton-Coupled Electron Transfer. The Royal Society of Chemistry; Cambridge, UK: 2012. pp. 1–157. (Catalysis Series). - DOI
-
- Britikov V.V., Bocharov E.V., Britikova E.V., Dergousova N.I., Kulikova O.G., Solovieva A.Y., Shipkov N.S., Varfolomeeva L.A., Tikhonova T.V., Timofeev V.I., et al. Unusual Cytochrome c552 from Thioalkalivibrio paradoxus: Solution NMR Structure and Interaction with Thiocyanate Dehydrogenase. Int. J. Mol. Sci. 2022;23:9969. doi: 10.3390/ijms23179969. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

