Apoptosis Evaluation in Circulating CD34+-Enriched Hematopoietic Stem and Progenitor Cells in Patients with Abnormally Increased Production of Endogenous Glucocorticoids in Course of Cushing's Syndrome
- PMID: 36555435
- PMCID: PMC9779045
- DOI: 10.3390/ijms232415794
Apoptosis Evaluation in Circulating CD34+-Enriched Hematopoietic Stem and Progenitor Cells in Patients with Abnormally Increased Production of Endogenous Glucocorticoids in Course of Cushing's Syndrome
Abstract
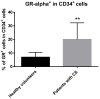
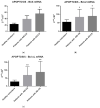
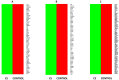
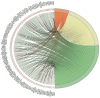
Abnormalities in hematological parameters of peripheral blood have been noted in patients with endogenous Cushing's Syndrome (CS) in the corticotropin (ACTH)-dependent and ACTH-independent forms. Nevertheless, the exact mechanism of glucocorticoids (GCs) action on human hematopoiesis is still not entirely clear. The aim of the study was to determine whether endogenous excessive production of GCs could affect apoptosis of CD34+ cells enriched in hematopoietic stem and progenitor cells (HSPCs) collected from the peripheral blood of newly diagnosed CS patients. Flow cytometry, Annexin-V enzyme-linked immunosorbent assay, TUNEL assay, real-time quantitative PCR, and microarray RNA/miRNA techniques were used to characterize CS patients' HSPCs. We found that the glucocorticoid receptor (GR) protein expression levels in CS were higher than in healthy controls. A complex analysis of apoptotic status of CS patients' HSPC cells showed that GCs significantly augmented apoptosis in peripheral blood-derived CD34+ cells and results obtained using different methods to detect early and late apoptosis in analyzed cell population were consistent. CS was also associated with significant upregulation in several members of the BCL-2 superfamily and other genes associated with apoptosis control. Furthermore, global gene expression analysis revealed significantly higher expression of genes associated with programmed cell death control in HSPCs from CS patients. These findings suggest that human endogenous GCs have a direct pro-apoptotic activity in hematopoietic CD34+ cells derived from CS subjects before treatment.
Keywords: BCL-2; Cushing’s Syndrome; RNA; apoptosis; glucocorticoids; hematopoietic stem and progenitor cells; microRNA; microarrays.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures









Similar articles
-
Glucocorticoid receptors and Cushing's disease.Mol Cell Endocrinol. 2002 Nov 29;197(1-2):69-72. doi: 10.1016/s0303-7207(02)00280-0. Mol Cell Endocrinol. 2002. PMID: 12431798 Review.
-
Expression of selected angiogenesis-related small microRNAs in patients with abnormally increased secretion of glucocorticoids.Endokrynol Pol. 2019;70(6):489-495. doi: 10.5603/EP.a2019.0061. Endokrynol Pol. 2019. PMID: 31909455
-
The Impact of Growth Hormone Therapy on the Apoptosis Assessment in CD34+ Hematopoietic Cells from Children with Growth Hormone Deficiency.Int J Mol Sci. 2017 Jan 7;18(1):111. doi: 10.3390/ijms18010111. Int J Mol Sci. 2017. PMID: 28067847 Free PMC article.
-
Decreased ligand affinity rather than glucocorticoid receptor down-regulation in patients with endogenous Cushing's syndrome.Eur J Endocrinol. 2000 May;142(5):472-6. doi: 10.1530/eje.0.1420472. Eur J Endocrinol. 2000. PMID: 10802524
-
New types of localization methods for adrenocorticotropic hormone-dependent Cushing's syndrome.Best Pract Res Clin Endocrinol Metab. 2021 Jan;35(1):101513. doi: 10.1016/j.beem.2021.101513. Epub 2021 Mar 10. Best Pract Res Clin Endocrinol Metab. 2021. PMID: 34045044 Review.
References
-
- Pitzalis C., Pipitone N., Bajocchi G., Hall M., Goulding N., Lee A., Kingsley G., Lanchbury J., Panayi G. Corticosteroids inhibit lymphocyte binding to endothelium and intercellular adhesion: An additional mechanism for their anti-inflammatory and immunosuppressive effect. J. Immunol. 1997;158:5007–5016. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

