Selective CB2 Receptor Agonist, HU-308, Reduces Systemic Inflammation in Endotoxin Model of Pneumonia-Induced Acute Lung Injury
- PMID: 36555499
- PMCID: PMC9779896
- DOI: 10.3390/ijms232415857
Selective CB2 Receptor Agonist, HU-308, Reduces Systemic Inflammation in Endotoxin Model of Pneumonia-Induced Acute Lung Injury
Abstract
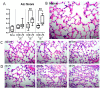
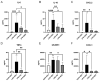
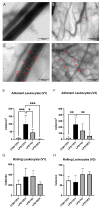
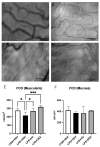
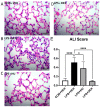
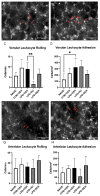
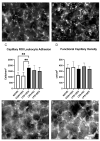
Acute respiratory distress syndrome (ARDS) and sepsis are risk factors contributing to mortality in patients with pneumonia. In ARDS, also termed acute lung injury (ALI), pulmonary immune responses lead to excessive pro-inflammatory cytokine release and aberrant alveolar neutrophil infiltration. Systemic spread of cytokines is associated with systemic complications including sepsis, multi-organ failure, and death. Thus, dampening pro-inflammatory cytokine release is a viable strategy to improve outcome. Activation of cannabinoid type II receptor (CB2) has been shown to reduce cytokine release in various in vivo and in vitro studies. Herein, we investigated the effect of HU-308, a specific CB2 agonist, on systemic and pulmonary inflammation in a model of pneumonia-induced ALI. C57Bl/6 mice received intranasal endotoxin or saline, followed by intravenous HU-308, dexamethasone, or vehicle. ALI was scored by histology and plasma levels of select inflammatory mediators were assessed by Luminex assay. Intravital microscopy (IVM) was performed to assess leukocyte adhesion and capillary perfusion in intestinal and pulmonary microcirculation. HU-308 and dexamethasone attenuated LPS-induced cytokine release and intestinal microcirculatory impairment. HU-308 modestly reduced ALI score, while dexamethasone abolished it. These results suggest administration of HU-308 can reduce systemic inflammation without suppressing pulmonary immune response in pneumonia-induced ALI and systemic inflammation.
Keywords: ARDS; acute lung injury; cannabinoid type II receptor (CB2); cytokines; inflammation; intravital microscopy; microcirculation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cannabidiol Reduces Systemic Immune Activation in Experimental Acute Lung Injury.Cannabis Cannabinoid Res. 2024 Oct;9(5):1301-1311. doi: 10.1089/can.2023.0039. Epub 2023 Oct 9. Cannabis Cannabinoid Res. 2024. PMID: 37815809
-
Activation of cannabinoid-2 receptor protects against Pseudomonas aeruginosa induced acute lung injury and inflammation.Respir Res. 2022 Dec 3;23(1):326. doi: 10.1186/s12931-022-02253-w. Respir Res. 2022. PMID: 36463179 Free PMC article.
-
The HDL from septic-ARDS patients with composition changes exacerbates pulmonary endothelial dysfunction and acute lung injury induced by cecal ligation and puncture (CLP) in mice.Respir Res. 2020 Nov 4;21(1):293. doi: 10.1186/s12931-020-01553-3. Respir Res. 2020. PMID: 33148285 Free PMC article.
-
Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury.Front Immunol. 2020 Aug 4;11:1722. doi: 10.3389/fimmu.2020.01722. eCollection 2020. Front Immunol. 2020. PMID: 32849610 Free PMC article. Review.
-
Ameliorating effects of berberine on sepsis-associated lung inflammation induced by lipopolysaccharide: molecular mechanisms and preclinical evidence.Pharmacol Rep. 2023 Aug;75(4):805-816. doi: 10.1007/s43440-023-00492-2. Epub 2023 May 15. Pharmacol Rep. 2023. PMID: 37184743 Review.
Cited by
-
The Impact of the CB2 Cannabinoid Receptor in Inflammatory Diseases: An Update.Molecules. 2024 Jul 18;29(14):3381. doi: 10.3390/molecules29143381. Molecules. 2024. PMID: 39064959 Free PMC article. Review.
-
Cell Death in Acute Organ Injury and Fibrosis.Int J Mol Sci. 2024 Apr 1;25(7):3930. doi: 10.3390/ijms25073930. Int J Mol Sci. 2024. PMID: 38612740 Free PMC article.
-
Complete Genome Analyses of a Novel Flexivirus with Unique Genome Organization and Three Endornaviruses Hosted by the Mycorrhizal Fungus Terfezia claveryi.Curr Microbiol. 2024 Jun 5;81(7):210. doi: 10.1007/s00284-024-03745-2. Curr Microbiol. 2024. PMID: 38837067
-
Intravital Microscopy of Lipopolysaccharide-Induced Inflammatory Changes in Different Organ Systems-A Scoping Review.Int J Mol Sci. 2023 Nov 15;24(22):16345. doi: 10.3390/ijms242216345. Int J Mol Sci. 2023. PMID: 38003533 Free PMC article.
-
Enantiomeric Agonists of the Type 2 Cannabinoid Receptor Reduce Retinal Damage during Proliferative Vitreoretinopathy and Inhibit Hyperactive Microglia In Vitro.ACS Pharmacol Transl Sci. 2024 Apr 25;7(5):1348-1363. doi: 10.1021/acsptsci.4c00014. eCollection 2024 May 10. ACS Pharmacol Transl Sci. 2024. PMID: 38751621 Free PMC article.
References
-
- Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A., Gattinoni L., Van Haren F.M.P., Larsson A., McAuley D.F., et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA—J. Am. Med. Assoc. 2016;315:788–800. doi: 10.1001/jama.2016.0291. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

