PKR-Mediated Phosphorylation of eIF2a and CHK1 Is Associated with Doxorubicin-Mediated Apoptosis in HCC1143 Triple-Negative Breast Cancer Cells
- PMID: 36555509
- PMCID: PMC9779813
- DOI: 10.3390/ijms232415872
PKR-Mediated Phosphorylation of eIF2a and CHK1 Is Associated with Doxorubicin-Mediated Apoptosis in HCC1143 Triple-Negative Breast Cancer Cells
Abstract
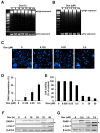
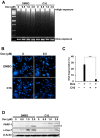
Triple-negative breast cancer is more aggressive than other types of breast cancer. Protein kinase R (PKR), which is activated by dsRNA, is known to play a role in doxorubicin-mediated apoptosis; however, its role in DNA damage-mediated apoptosis is not well understood. In this study, we investigated the roles of PKR and its downstream players in doxorubicin-treated HCC1143 triple-negative breast cancer cells. Doxorubicin treatment induces DNA damage and apoptosis. Interestingly, doxorubicin treatment induced the phosphorylation of eukaryotic initiation factor 2 alpha (eIF2α) via PKR, whereas the inhibition of PKR with inhibitor C16 reduced eIF2α phosphorylation. Under these conditions, doxorubicin-mediated DNA fragmentation, cell death, and poly(ADP ribose) polymerase and caspase 7 levels were recovered. In addition, phosphorylation of checkpoint kinase 1 (CHK1), which is known to be involved in doxorubicin-mediated DNA damage, was increased by doxorubicin treatment, but blocked by PKR inhibition. Protein translation was downregulated by doxorubicin treatment and upregulated by blocking PKR phosphorylation. These results suggest that PKR activation induces apoptosis by increasing the phosphorylation of eIF2α and CHK1 and decreasing the global protein translation in doxorubicin-treated HCC1143 triple-negative breast cancer cells.
Keywords: CHK1; ER; PKR; apoptosis; doxorubicin; eIF2α; triple-negative breast cancer.
Conflict of interest statement
All authors declare no competing interests.
Figures


Similar articles
-
Severe acute respiratory syndrome coronavirus triggers apoptosis via protein kinase R but is resistant to its antiviral activity.J Virol. 2009 Mar;83(5):2298-309. doi: 10.1128/JVI.01245-08. Epub 2008 Dec 24. J Virol. 2009. PMID: 19109397 Free PMC article.
-
Doxorubicin bypasses the cytoprotective effects of eIF2α phosphorylation and promotes PKR-mediated cell death.Cell Death Differ. 2011 Jan;18(1):145-54. doi: 10.1038/cdd.2010.76. Epub 2010 Jun 18. Cell Death Differ. 2011. PMID: 20559319 Free PMC article.
-
Human Herpesvirus 6A Exhibits Restrictive Propagation with Limited Activation of the Protein Kinase R-eIF2α Stress Pathway.J Virol. 2017 Apr 13;91(9):e02120-16. doi: 10.1128/JVI.02120-16. Print 2017 May 1. J Virol. 2017. PMID: 28202752 Free PMC article.
-
Involvement of double-stranded RNA-dependent protein kinase and phosphorylation of eukaryotic initiation factor-2alpha in neuronal degeneration.J Neurochem. 2002 Dec;83(5):1215-25. doi: 10.1046/j.1471-4159.2002.01237.x. J Neurochem. 2002. PMID: 12437593
-
Increased expression of the dsRNA-activated protein kinase PKR in breast cancer promotes sensitivity to doxorubicin.PLoS One. 2012;7(9):e46040. doi: 10.1371/journal.pone.0046040. Epub 2012 Sep 24. PLoS One. 2012. PMID: 23029376 Free PMC article.
Cited by
-
Exploiting Translation Machinery for Cancer Therapy: Translation Factors as Promising Targets.Int J Mol Sci. 2024 Oct 9;25(19):10835. doi: 10.3390/ijms251910835. Int J Mol Sci. 2024. PMID: 39409166 Free PMC article. Review.
-
Smooth Muscle Cell Phenotypic Switch Induced by Traditional Cigarette Smoke Condensate: A Holistic Overview.Int J Mol Sci. 2023 Mar 29;24(7):6431. doi: 10.3390/ijms24076431. Int J Mol Sci. 2023. PMID: 37047404 Free PMC article.
-
Checkpoint kinase 1 in colorectal cancer: Upregulation of expression and promotion of cell proliferation.World J Clin Oncol. 2025 Mar 24;16(3):101725. doi: 10.5306/wjco.v16.i3.101725. World J Clin Oncol. 2025. PMID: 40130044 Free PMC article.
-
Harnessing p53 for targeted cancer therapy: new advances and future directions.Transcription. 2025 Feb;16(1):3-46. doi: 10.1080/21541264.2025.2452711. Epub 2025 Mar 3. Transcription. 2025. PMID: 40031988 Free PMC article. Review.
-
Unravelling the role of protein kinase R (PKR) in neurodegenerative disease: a review.Mol Biol Rep. 2025 Apr 9;52(1):377. doi: 10.1007/s11033-025-10484-5. Mol Biol Rep. 2025. PMID: 40205152 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

