µ-Opioid Receptors Expressed by Intrinsically Photosensitive Retinal Ganglion Cells Contribute to Morphine-Induced Behavioral Sensitization
- PMID: 36555511
- PMCID: PMC9781919
- DOI: 10.3390/ijms232415870
µ-Opioid Receptors Expressed by Intrinsically Photosensitive Retinal Ganglion Cells Contribute to Morphine-Induced Behavioral Sensitization
Abstract
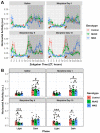
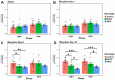
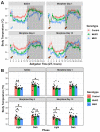
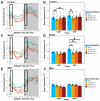
Opioid drugs are the most effective tools for treating moderate to severe pain. Despite their analgesic efficacy, long-term opioid use can lead to drug tolerance, addiction, and sleep/wake disturbances. While the link between opioids and sleep/wake problems is well-documented, the mechanism underlying opioid-related sleep/wake problems remains largely unresolved. Importantly, intrinsically photosensitive retinal ganglion cells (ipRGCs), the cells that transmit environmental light/dark information to the brain's sleep/circadian centers to regulate sleep/wake behavior, express μ-opioid receptors (MORs). In this study, we explored the potential contribution of ipRGCs to opioid-related sleep/circadian disruptions. Using implanted telemetry transmitters, we measured changes in horizontal locomotor activity and body temperature in mice over the course of a chronic morphine paradigm. Mice lacking MORs expressed by ipRGCs (McKO) exhibited reduced morphine-induced behavioral activation/sensitization compared with control littermates with normal patterns of MOR expression. Contrastingly, mice lacking MORs globally (MKO) did not acquire morphine-induced locomotor activation/sensitization. Control mice also showed morphine-induced hypothermia in both the light and dark phases, while McKO littermates only exhibited morphine-induced hypothermia in the dark. Interestingly, only control animals appeared to acquire tolerance to morphine's hypothermic effect. Morphine, however, did not acutely decrease the body temperature of MKO mice. These findings support the idea that MORs expressed by ipRGCs could contribute to opioid-related sleep/wake problems and thermoregulatory changes.
Keywords: addiction; behavioral sensitization; hypothermia; opioids; photoentrainment; retina; sleep/wake; μ-opioid receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A retinal contribution to opioid-induced sleep disorders?Front Neurosci. 2022 Aug 5;16:981939. doi: 10.3389/fnins.2022.981939. eCollection 2022. Front Neurosci. 2022. PMID: 35992901 Free PMC article. Review.
-
Endogenous opioid signaling in the retina modulates sleep/wake activity in mice.Neurobiol Sleep Circadian Rhythms. 2022 Jun 26;13:100078. doi: 10.1016/j.nbscr.2022.100078. eCollection 2022 Nov. Neurobiol Sleep Circadian Rhythms. 2022. PMID: 35800978 Free PMC article.
-
μ-Opioid Receptor Activation Directly Modulates Intrinsically Photosensitive Retinal Ganglion Cells.Neuroscience. 2019 Jun 1;408:400-417. doi: 10.1016/j.neuroscience.2019.04.005. Epub 2019 Apr 11. Neuroscience. 2019. PMID: 30981862 Free PMC article.
-
μ-Opioid receptors in primary sensory neurons are essential for opioid analgesic effect on acute and inflammatory pain and opioid-induced hyperalgesia.J Physiol. 2019 Mar;597(6):1661-1675. doi: 10.1113/JP277428. Epub 2019 Jan 16. J Physiol. 2019. PMID: 30578671 Free PMC article.
-
The mechanism of μ-opioid receptor (MOR)-TRPV1 crosstalk in TRPV1 activation involves morphine anti-nociception, tolerance and dependence.Channels (Austin). 2015;9(5):235-43. doi: 10.1080/19336950.2015.1069450. Epub 2015 Jul 15. Channels (Austin). 2015. PMID: 26176938 Free PMC article. Review.
Cited by
-
Morphine pharmacokinetics and opioid transporter expression at the blood-retina barrier of male and female mice.Front Pharmacol. 2023 Jun 14;14:1206104. doi: 10.3389/fphar.2023.1206104. eCollection 2023. Front Pharmacol. 2023. PMID: 37388441 Free PMC article.
-
Dopamine enhances GABAA receptor-mediated current amplitude in a subset of intrinsically photosensitive retinal ganglion cells.bioRxiv [Preprint]. 2023 Dec 12:2023.12.11.571141. doi: 10.1101/2023.12.11.571141. bioRxiv. 2023. Update in: J Neurophysiol. 2024 Aug 1;132(2):501-513. doi: 10.1152/jn.00457.2023. PMID: 38168350 Free PMC article. Updated. Preprint.
-
Dopamine enhances GABAA receptor-mediated current amplitude in a subset of intrinsically photosensitive retinal ganglion cells.J Neurophysiol. 2024 Aug 1;132(2):501-513. doi: 10.1152/jn.00457.2023. Epub 2024 Jul 3. J Neurophysiol. 2024. PMID: 38958282 Free PMC article.
References
-
- Chrobok A.I., Krause D., Winter C., Plörer D., Martin G., Koller G., Adorjan K., Canolli M., Adam R., Wagner E.M., et al. Sleeping Patterns in Patients with Opioid Use Disorder: Effects of Opioid Maintenance Treatment and Detoxification. J. Psychoact. Drugs. 2020;52:203–210. doi: 10.1080/02791072.2020.1751900. - DOI - PubMed
-
- Dimsdale J.E., Norman D., DeJardin D., Wallace M. The Effect of Opioids on Sleep Architecture. J. Clin. Sleep Med. 2007;3:4. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

