SH2 Domains: Folding, Binding and Therapeutical Approaches
- PMID: 36555586
- PMCID: PMC9783222
- DOI: 10.3390/ijms232415944
SH2 Domains: Folding, Binding and Therapeutical Approaches
Abstract
SH2 (Src Homology 2) domains are among the best characterized and most studied protein-protein interaction (PPIs) modules able to bind and recognize sequences presenting a phosphorylated tyrosine. This post-translational modification is a key regulator of a plethora of physiological and molecular pathways in the eukaryotic cell, so SH2 domains possess a fundamental role in cell signaling. Consequently, several pathologies arise from the dysregulation of such SH2-domains mediated PPIs. In this review, we recapitulate the current knowledge about the structural, folding stability, and binding properties of SH2 domains and their roles in molecular pathways and pathogenesis. Moreover, we focus attention on the different strategies employed to modulate/inhibit SH2 domains binding. Altogether, the information gathered points to evidence that pharmacological interest in SH2 domains is highly strategic to developing new therapeutics. Moreover, a deeper understanding of the molecular determinants of the thermodynamic stability as well as of the binding properties of SH2 domains appears to be fundamental in order to improve the possibility of preventing their dysregulated interactions.
Keywords: Src Homology 2; phosphotyrosine; protein-protein interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


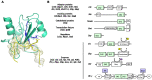
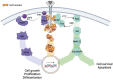
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

