A Novel Senescence-Specific Gene (ZmSAG39) Negatively Regulates Darkness and Drought Responses in Maize
- PMID: 36555622
- PMCID: PMC9785699
- DOI: 10.3390/ijms232415984
A Novel Senescence-Specific Gene (ZmSAG39) Negatively Regulates Darkness and Drought Responses in Maize
Abstract
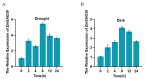
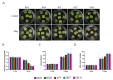
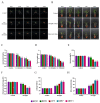
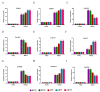
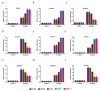
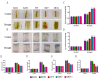
The papain-like cysteine proteases (PLCPs) is a subfamily of cysteine proteases that plays an important role in leaf senescence, and some of its members are involved in the regulation of plant growth and development under stress. In this study, we cloned a new gene, ZmSAG39, from maize. Expression profile analysis showed that ZmSAG39 was induced by darkness and drought treatments. In addition, the ZmSAG39 overexpression in maize accelerated the senescence of maize leaves under darkness and drought treatments. However, the knockout of ZmSAG39 in maize enhanced the resistance of maize to darkness and drought stresses and reduced the degree of senescence of maize leaves. Under drought stress, compared with WT plants, the knockout lines had a higher seed germination rate, seedling survival rate and chlorophyll content, and lower reactive oxygen species (ROS) level and malondialdehyde (MDA) content. In addition, quantitative real-time PCR (qRT-PCR) analysis showed that ZmSAG39 negatively regulated some stress-related genes but positively regulated senescence-related genes under darkness and drought stress conditions. To summarize, these results indicate that ZmSAG39 is a senescence-related gene and plays a negative role in response to darkness and drought stresses. This study laid a theoretical foundation for the innovation of maize germplasm resources with high quality, high yield and strong stress resistance.
Keywords: ZmSAG39; darkness stress; drought stress; leaf senescence; maize.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Genetic, molecular and physiological crosstalk during drought tolerance in maize (Zea mays): pathways to resilient agriculture.Planta. 2024 Aug 28;260(4):81. doi: 10.1007/s00425-024-04517-9. Planta. 2024. PMID: 39196449 Review.
-
Maize ZmWRKY71 gene positively regulates drought tolerance through reactive oxygen species homeostasis.Plant Physiol Biochem. 2025 Feb;219:109399. doi: 10.1016/j.plaphy.2024.109399. Epub 2024 Dec 9. Plant Physiol Biochem. 2025. PMID: 39689610
-
A novel NAC transcription factor ZmNAC55 negatively regulates drought stress in Zea mays.Plant Physiol Biochem. 2024 Sep;214:108938. doi: 10.1016/j.plaphy.2024.108938. Epub 2024 Jul 14. Plant Physiol Biochem. 2024. PMID: 39067103
-
ZmASR3 from the Maize ASR Gene Family Positively Regulates Drought Tolerance in Transgenic Arabidopsis.Int J Mol Sci. 2019 May 8;20(9):2278. doi: 10.3390/ijms20092278. Int J Mol Sci. 2019. PMID: 31072025 Free PMC article.
-
Transcriptional regulatory networks in response to drought stress and rewatering in maize (Zea mays L.).Mol Genet Genomics. 2021 Nov;296(6):1203-1219. doi: 10.1007/s00438-021-01820-y. Epub 2021 Oct 3. Mol Genet Genomics. 2021. PMID: 34601650 Review.
Cited by
-
Molecular mapping and validation of quantitative trait loci for content of micronutrients in wheat grain.Front Plant Sci. 2025 Jan 17;15:1522465. doi: 10.3389/fpls.2024.1522465. eCollection 2024. Front Plant Sci. 2025. PMID: 39898268 Free PMC article.
-
Overexpression of soybean GmDHN9 gene enhances drought resistance of transgenic Arabidopsis.GM Crops Food. 2024 Dec 31;15(1):118-129. doi: 10.1080/21645698.2024.2327116. Epub 2024 Apr 2. GM Crops Food. 2024. PMID: 38564429 Free PMC article.
-
A Cinnamate 4-HYDROXYLASE1 from Safflower Promotes Flavonoids Accumulation and Stimulates Antioxidant Defense System in Arabidopsis.Int J Mol Sci. 2023 Mar 11;24(6):5393. doi: 10.3390/ijms24065393. Int J Mol Sci. 2023. PMID: 36982470 Free PMC article.
-
Genetic, molecular and physiological crosstalk during drought tolerance in maize (Zea mays): pathways to resilient agriculture.Planta. 2024 Aug 28;260(4):81. doi: 10.1007/s00425-024-04517-9. Planta. 2024. PMID: 39196449 Review.
-
ZmNF-YB10, a maize NF-Y transcription factor, positively regulates drought and salt stress response in Arabidopsis thaliana.GM Crops Food. 2025 Dec;16(1):28-45. doi: 10.1080/21645698.2024.2438421. Epub 2024 Dec 24. GM Crops Food. 2025. PMID: 39718137 Free PMC article.
References
-
- Feng X., Liu L., Li Z., Sun F., Wu X., Hao D., Hao H., Jing H.C. Potential interaction between autophagy and auxin during maize leaf senescence implicated by population genetics and high resolution gene expression profiling. J. Exp. Bot. 2021;10:3554–3568. doi: 10.1093/jxb/erab094. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

