The Reactions of N,N'-Diphenyldithiomalondiamide with Arylmethylidene Meldrum's Acids
- PMID: 36555639
- PMCID: PMC9785638
- DOI: 10.3390/ijms232415997
The Reactions of N,N'-Diphenyldithiomalondiamide with Arylmethylidene Meldrum's Acids
Abstract
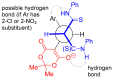
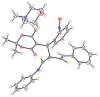
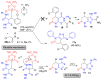
The Michael addition reaction between dithiomalondianilide (N,N'-diphenyldithiomalondiamide) and arylmethylidene Meldrum's acids, accompanied by subsequent heterocyclization, was investigated along with factors affecting the mixture composition of the obtained products. The plausible mechanism includes the formation of stable Michael adducts which, under the studied conditions, undergo further transformations to yield corresponding N-methylmorpholinium 4-aryl-6-oxo-3-(N-phenylthio-carbamoyl)-1,4,5,6-tetrahydropyridin-2-thiolates and their oxidation derivatives, 4,5-dihydro-3H-[1,2]dithiolo[3,4-b]pyridin-6(7H)-ones. The structure of one such product, N-methylmorpholinium 2,2-dimethyl-5-(1-(2-nitrophenyl)-3-(phenylamino)-2-(N-phenylthiocarbamoyl)-3-thioxopropyl)-4-oxo-4H-1,3-dioxin-6-olate, was confirmed via X-ray crystallography.
Keywords: Meldrum’s acid; Michael adducts; active methylene compounds; dithiomalonamides; heterocyclization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


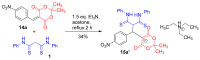

Similar articles
-
Alkyl 4-Aryl-6-amino-7- phenyl-3-(phenylimino)-4,7-dihydro- 3H-[1,2]dithiolo[3,4-b]pyridine-5-carboxylates: Synthesis and Agrochemical Studies.Molecules. 2023 Jan 6;28(2):609. doi: 10.3390/molecules28020609. Molecules. 2023. PMID: 36677666 Free PMC article.
-
Design and synthesis of pyrido[2,1-b][1,3,5]thiadiazine library via uncatalyzed Mannich-type reaction.ACS Comb Sci. 2014 Oct 13;16(10):543-50. doi: 10.1021/co5000807. Epub 2014 Sep 23. ACS Comb Sci. 2014. PMID: 25191927
-
Kinetic study of electrochemically induced michael reactions of o-quinones with Meldrum's acid derivatives. Synthesis of highly oxygenated catechols.J Org Chem. 2008 May 2;73(9):3428-34. doi: 10.1021/jo800115n. Epub 2008 Apr 9. J Org Chem. 2008. PMID: 18396907
-
Meldrum's acid and related compounds in the synthesis of natural products and analogs.Chem Soc Rev. 2008 Apr;37(4):789-811. doi: 10.1039/b716020h. Epub 2008 Feb 12. Chem Soc Rev. 2008. PMID: 18362984 Review.
-
One hundred years of Meldrum's acid: advances in the synthesis of pyridine and pyrimidine derivatives.Mol Divers. 2009 Nov;13(4):399-419. doi: 10.1007/s11030-009-9136-x. Epub 2009 Apr 21. Mol Divers. 2009. PMID: 19381852 Review.
Cited by
-
6-Amino-4-aryl-7-phenyl-3-(phenylimino)-4,7-dihydro-3H-[1,2]dithiolo[3,4-b]pyridine-5-carboxamides: Synthesis, Biological Activity, Quantum Chemical Studies and In Silico Docking Studies.Int J Mol Sci. 2024 Jan 7;25(2):769. doi: 10.3390/ijms25020769. Int J Mol Sci. 2024. PMID: 38255843 Free PMC article.
-
Alkyl 4-Aryl-6-amino-7- phenyl-3-(phenylimino)-4,7-dihydro- 3H-[1,2]dithiolo[3,4-b]pyridine-5-carboxylates: Synthesis and Agrochemical Studies.Molecules. 2023 Jan 6;28(2):609. doi: 10.3390/molecules28020609. Molecules. 2023. PMID: 36677666 Free PMC article.
References
-
- Becker J., Stidsen C.E. Recent developments in the synthesis and chemistry of 2(1H)-pyridinethiones and related compounds. Sulfur Rep. 1988;8:105–146. doi: 10.1080/01961778808046175. - DOI
-
- Litvinov V.P., Rodinovskaya L.A., Sharanin Y.A., Shestopalov A.M., Senning A. Advances in the chemistry of 3-cyanopyridin-2(1H)-ones, -thiones, and -selenones. J. Sulfur Chem. 1992;13:1–142. doi: 10.1080/01961779208048951. - DOI
-
- Litvinov V.P. Advances in the chemistry of hydrogenated 3-cyanopyridine-2(1H)-thiones and -selenones. Phosphorus Sulfur Silicon Relat. Elem. 1993;74:139–156. doi: 10.1080/10426509308038105. - DOI
-
- Litvinov V.P. Partially hydrogenated pyridinechalcogenones. Russ. Chem. Bull. 1998;47:2053–2073. doi: 10.1007/BF02494257. - DOI
-
- Litvinov V.P., Krivokolysko S.G., Dyachenko V.D. Synthesis and properties of 3-cyanopyridine-2(1H)-chalcogenones. Chem. Heterocycl. Compd. 1999;35:509–540. doi: 10.1007/BF02324634. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

