β-Caryophyllene Acts as a Ferroptosis Inhibitor to Ameliorate Experimental Colitis
- PMID: 36555694
- PMCID: PMC9784863
- DOI: 10.3390/ijms232416055
β-Caryophyllene Acts as a Ferroptosis Inhibitor to Ameliorate Experimental Colitis
Abstract
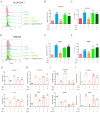
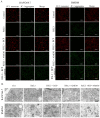
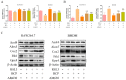
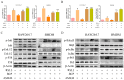
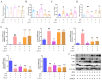
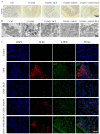
Macrophage infiltration is one of the main pathological features of ulcerative colitis (UC) and ferroptosis is a type of nonapoptotic cell death, connecting oxidative stress and inflammation. However, whether ferroptosis occurs in the colon macrophages of UC mice and whether targeting macrophage ferroptosis is an effective approach for UC treatment remain unclear. The present study revealed that macrophage lipid peroxidation was observed in the colon of UC mice. Subsequently, we screened several main components of essential oil from Artemisia argyi and found that β-caryophyllene (BCP) had a good inhibitory effect on macrophage lipid peroxidation. Additionally, ferroptotic macrophages were found to increase the mRNA expression of tumor necrosis factor alpha (Tnf-α) and prostaglandin-endoperoxide synthase 2 (Ptgs2), while BCP can reverse the effects of inflammation activated by ferroptosis. Further molecular mechanism studies revealed that BCP activated the type 2 cannabinoid receptor (CB2R) to inhibit macrophage ferroptosis and its induced inflammatory response both in vivo and in vitro. Taken together, BCP potentially ameliorated experimental colitis inflammation by inhibiting macrophage ferroptosis. These results revealed that macrophage ferroptosis is a potential therapeutic target for UC and identified a novel mechanism of BCP in ameliorating experimental colitis.
Keywords: ferroptosis; inflammation; macrophage; type 2 cannabinoid receptor; ulcerative colitis; β-caryophyllene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

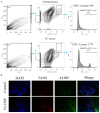
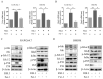
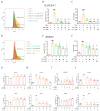

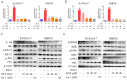
References
-
- Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., Panaccione R., Ghosh S., Wu J.C.Y., Chan F.K.L., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

