Labeled TEMPO-Oxidized Mannan Differentiates Binding Profiles within the Collectin Families
- PMID: 36555720
- PMCID: PMC9786299
- DOI: 10.3390/ijms232416067
Labeled TEMPO-Oxidized Mannan Differentiates Binding Profiles within the Collectin Families
Abstract
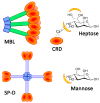
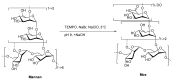
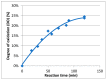
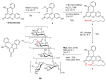
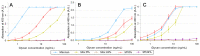
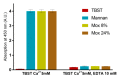
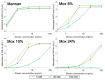
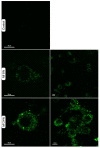
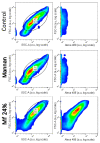
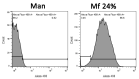
Establishing the rapid and accurate diagnosis of sepsis is a key component to the improvement of clinical outcomes. The ability of analytical platforms to rapidly detect pathogen-associated molecular patterns (PAMP) in blood could provide a powerful host-independent biomarker of sepsis. A novel concept was investigated based on the idea that a pre-bound and fluorescent ligand could be released from lectins in contact with high-affinity ligands (such as PAMPs). To create fluorescent ligands with precise avidity, the kinetically followed TEMPO oxidation of yeast mannan and carbodiimide coupling were used. The chemical modifications led to decreases in avidity between mannan and human collectins, such as the mannan-binding lectin (MBL) and human surfactant protein D (SP-D), but not in porcine SP-D. Despite this effect, these fluorescent derivatives were captured by human lectins using highly concentrated solutions. The resulting fluorescent beads were exposed to different solutions, and the results showed that displacements occur in contact with higher affinity ligands, proving that two-stage competition processes can occur in collectin carbohydrate recognition mechanisms. Moreover, the fluorescence loss depends on the discrepancy between the respective avidities of the recognized ligand and the fluorescent mannan. Chemically modulated fluorescent ligands associated with a diversity of collectins may lead to the creation of diagnostic tools suitable for multiplex array assays and the identification of high-avidity ligands.
Keywords: avidity; binding; fluorescent; lectin; mannan; oxidation; surfactant protein D; tempo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


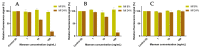

Similar articles
-
Collectins and collectin receptors in innate immunity.APMIS Suppl. 2000;100:1-59. APMIS Suppl. 2000. PMID: 11021254 Review.
-
Nucleic acid is a novel ligand for innate, immune pattern recognition collectins surfactant proteins A and D and mannose-binding lectin.J Biol Chem. 2004 Jul 30;279(31):32728-36. doi: 10.1074/jbc.M403763200. Epub 2004 May 15. J Biol Chem. 2004. PMID: 15145932
-
Purification and characterization of a bovine serum lectin (CL-43) with structural homology to conglutinin and SP-D and carbohydrate specificity similar to mannan-binding protein.J Biol Chem. 1993 May 15;268(14):10120-5. J Biol Chem. 1993. PMID: 8486682
-
Increasing antiviral activity of surfactant protein d trimers by introducing residues from bovine serum collectins: dissociation of mannan-binding and antiviral activity.Scand J Immunol. 2010 Jul;72(1):22-30. doi: 10.1111/j.1365-3083.2010.02409.x. Scand J Immunol. 2010. PMID: 20591072 Free PMC article.
-
Structure and function of collectins: humoral C-type lectins with collagenous regions.Behring Inst Mitt. 1993 Dec;(93):224-35. Behring Inst Mitt. 1993. PMID: 8172571 Review.
References
-
- Colin Hughes R. Lectins as Cell Adhesion Molecules. Curr. Opin. Struct. Biol. 1992;2:687–692. doi: 10.1016/0959-440X(92)90202-I. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

