Histological Methods to Assess Skeletal Muscle Degeneration and Regeneration in Duchenne Muscular Dystrophy
- PMID: 36555721
- PMCID: PMC9786356
- DOI: 10.3390/ijms232416080
Histological Methods to Assess Skeletal Muscle Degeneration and Regeneration in Duchenne Muscular Dystrophy
Abstract
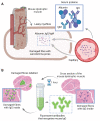
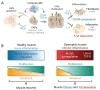
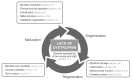
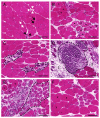
Duchenne muscular dystrophy (DMD) is a progressive disease caused by the loss of function of the protein dystrophin. This protein contributes to the stabilisation of striated cells during contraction, as it anchors the cytoskeleton with components of the extracellular matrix through the dystrophin-associated protein complex (DAPC). Moreover, absence of the functional protein affects the expression and function of proteins within the DAPC, leading to molecular events responsible for myofibre damage, muscle weakening, disability and, eventually, premature death. Presently, there is no cure for DMD, but different treatments help manage some of the symptoms. Advances in genetic and exon-skipping therapies are the most promising intervention, the safety and efficiency of which are tested in animal models. In addition to in vivo functional tests, ex vivo molecular evaluation aids assess to what extent the therapy has contributed to the regenerative process. In this regard, the later advances in microscopy and image acquisition systems and the current expansion of antibodies for immunohistological evaluation together with the development of different spectrum fluorescent dyes have made histology a crucial tool. Nevertheless, the complexity of the molecular events that take place in dystrophic muscles, together with the rise of a multitude of markers for each of the phases of the process, makes the histological assessment a challenging task. Therefore, here, we summarise and explain the rationale behind different histological techniques used in the literature to assess degeneration and regeneration in the field of dystrophinopathies, focusing especially on those related to DMD.
Keywords: Duchenne muscular dystrophy; animal model; degeneration; dystrophin; histology; immunofluorescence; immunohistology; myofibre; regeneration; skeletal muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The X-linked Becker muscular dystrophy (bmx) mouse models Becker muscular dystrophy via deletion of murine dystrophin exons 45-47.J Cachexia Sarcopenia Muscle. 2023 Apr;14(2):940-954. doi: 10.1002/jcsm.13171. Epub 2023 Jan 11. J Cachexia Sarcopenia Muscle. 2023. PMID: 36628607 Free PMC article.
-
Long-Term Protective Effect of Human Dystrophin Expressing Chimeric (DEC) Cell Therapy on Amelioration of Function of Cardiac, Respiratory and Skeletal Muscles in Duchenne Muscular Dystrophy.Stem Cell Rev Rep. 2022 Dec;18(8):2872-2892. doi: 10.1007/s12015-022-10384-2. Epub 2022 May 19. Stem Cell Rev Rep. 2022. PMID: 35590083 Free PMC article.
-
Alterations in Notch signalling in skeletal muscles from mdx and dko dystrophic mice and patients with Duchenne muscular dystrophy.Exp Physiol. 2014 Apr;99(4):675-87. doi: 10.1113/expphysiol.2013.077255. Epub 2014 Jan 17. Exp Physiol. 2014. PMID: 24443351
-
Disrupted Calcium Homeostasis in Duchenne Muscular Dystrophy: A Common Mechanism behind Diverse Consequences.Int J Mol Sci. 2021 Oct 13;22(20):11040. doi: 10.3390/ijms222011040. Int J Mol Sci. 2021. PMID: 34681707 Free PMC article. Review.
-
Chimeric Cell Therapies as a Novel Approach for Duchenne Muscular Dystrophy (DMD) and Muscle Regeneration.Biomolecules. 2024 May 13;14(5):575. doi: 10.3390/biom14050575. Biomolecules. 2024. PMID: 38785982 Free PMC article. Review.
Cited by
-
An Updated Analysis of Exon-Skipping Applicability for Duchenne Muscular Dystrophy Using the UMD-DMD Database.Genes (Basel). 2024 Nov 20;15(11):1489. doi: 10.3390/genes15111489. Genes (Basel). 2024. PMID: 39596689 Free PMC article. Review.
-
Lipin1 as a therapeutic target for respiratory insufficiency of duchenne muscular dystrophy.Front Physiol. 2024 Nov 12;15:1477976. doi: 10.3389/fphys.2024.1477976. eCollection 2024. Front Physiol. 2024. PMID: 39600918 Free PMC article.
-
Soluble Activin Receptor Type IIB Improves Muscle Regeneration Following Crotalus atrox Venom-Induced Damage.Toxins (Basel). 2025 Jan 28;17(2):59. doi: 10.3390/toxins17020059. Toxins (Basel). 2025. PMID: 39998076 Free PMC article.
-
Accumulation of Dystrophin-Positive Muscle Fibers and Improvement of Neuromuscular Junctions in mdx Mouse Muscles after Bone Marrow Transplantation under Different Conditions.Int J Mol Sci. 2023 May 17;24(10):8892. doi: 10.3390/ijms24108892. Int J Mol Sci. 2023. PMID: 37240237 Free PMC article.
-
Extracellular Matrix Proteomics: The mdx-4cv Mouse Diaphragm as a Surrogate for Studying Myofibrosis in Dystrophinopathy.Biomolecules. 2023 Jul 12;13(7):1108. doi: 10.3390/biom13071108. Biomolecules. 2023. PMID: 37509144 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

