Photobiomodulation Attenuated Cognitive Dysfunction and Neuroinflammation in a Prenatal Valproic Acid-Induced Autism Spectrum Disorder Mouse Model
- PMID: 36555737
- PMCID: PMC9785820
- DOI: 10.3390/ijms232416099
Photobiomodulation Attenuated Cognitive Dysfunction and Neuroinflammation in a Prenatal Valproic Acid-Induced Autism Spectrum Disorder Mouse Model
Abstract
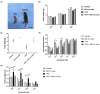
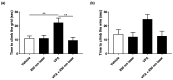
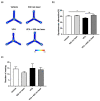
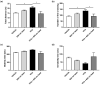
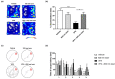
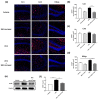
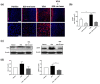
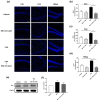
Autism spectrum disorder (ASD) is a neurodevelopmental condition characterized by social communication and interaction disorders, as well as repetitive and restrictive behaviors. To date, no effective treatment strategies have been identified. However, photobiomodulation (PBM) is emerging as a promising treatment for neurological and neuropsychiatric disorders. We used mice exposed to valproic acid (VPA) as a model of ASD and found that pathological behavioral and histological changes that may have been induced by VPA were attenuated by PBM treatment. Pregnant mice that had been exposed to VPA were treated with PBM three times. Thereafter, we evaluated the offspring for developmental disorders, motor function, hyperactivity, repetitive behaviors, and cognitive impairment. PBM attenuated many of the pathological behaviors observed in the VPA-induced ASD mouse model. In addition, pathophysiological analyses confirmed that the increase in activated microglia and astrocytes observed in the VPA-induced ASD mouse model was attenuated by PBM treatment. This suggests that PBM can counteract the behavioral changes caused by neuroinflammation in ASD. Therefore, our data show that PBM has therapeutic potential and may reduce the prevalence of neurodevelopmental disorders such as ASD.
Keywords: autism spectrum disorder; cognitive function; neuroinflammation; photobiomodulation; valproic acid.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Benefits of Fenofibrate in prenatal valproic acid-induced autism spectrum disorder related phenotype in rats.Brain Res Bull. 2019 Apr;147:36-46. doi: 10.1016/j.brainresbull.2019.02.003. Epub 2019 Feb 12. Brain Res Bull. 2019. PMID: 30769127
-
Ameliorating effect of pioglitazone on prenatal valproic acid-induced behavioral and neurobiological abnormalities in autism spectrum disorder in rats.Pharmacol Biochem Behav. 2024 Apr;237:173721. doi: 10.1016/j.pbb.2024.173721. Epub 2024 Feb 1. Pharmacol Biochem Behav. 2024. PMID: 38307465
-
Autism spectrum disorder-like behaviors induced by hyper-glutamatergic NMDA receptor signaling through hypo-serotonergic 5-HT1A receptor signaling in the prefrontal cortex in mice exposed to prenatal valproic acid.Neuropsychopharmacology. 2025 Apr;50(5):739-750. doi: 10.1038/s41386-024-02004-z. Epub 2024 Oct 11. Neuropsychopharmacology. 2025. PMID: 39394255 Free PMC article.
-
Prenatal valproate in rodents as a tool to understand the neural underpinnings of social dysfunctions in autism spectrum disorder.Neuropharmacology. 2019 Nov 15;159:107477. doi: 10.1016/j.neuropharm.2018.12.024. Epub 2019 Jan 9. Neuropharmacology. 2019. PMID: 30639388 Review.
-
Emerging mechanisms of valproic acid-induced neurotoxic events in autism and its implications for pharmacological treatment.Biomed Pharmacother. 2021 May;137:111322. doi: 10.1016/j.biopha.2021.111322. Epub 2021 Feb 16. Biomed Pharmacother. 2021. PMID: 33761592 Review.
Cited by
-
Photobiomodulation: shining a light on depression.Theranostics. 2025 Jan 1;15(2):362-383. doi: 10.7150/thno.104502. eCollection 2025. Theranostics. 2025. PMID: 39744683 Free PMC article. Review.
-
Prebiotic diet normalizes aberrant immune and behavioral phenotypes in a mouse model of autism spectrum disorder.Acta Pharmacol Sin. 2024 Aug;45(8):1591-1603. doi: 10.1038/s41401-024-01268-x. Epub 2024 Apr 8. Acta Pharmacol Sin. 2024. PMID: 38589690 Free PMC article.
-
Transcranial photobiomodulation in children aged 2-6 years: a randomized sham-controlled clinical trial assessing safety, efficacy, and impact on autism spectrum disorder symptoms and brain electrophysiology.Front Neurol. 2024 Apr 26;15:1221193. doi: 10.3389/fneur.2024.1221193. eCollection 2024. Front Neurol. 2024. PMID: 38737349 Free PMC article.
-
Unlocking the potential of photobiomodulation therapy for brain neurovascular coupling: The biological effects and medical applications.J Cereb Blood Flow Metab. 2025 May;45(5):800-830. doi: 10.1177/0271678X241311695. Epub 2025 Jan 7. J Cereb Blood Flow Metab. 2025. PMID: 39763390 Free PMC article. Review.
-
The Anti-Inflammatory Effect of Multi-Wavelength Light-Emitting Diode Irradiation Attenuates Dry Eye Symptoms in a Scopolamine-Induced Mouse Model of Dry Eye.Int J Mol Sci. 2023 Dec 14;24(24):17493. doi: 10.3390/ijms242417493. Int J Mol Sci. 2023. PMID: 38139321 Free PMC article.
References
-
- Edition F. Diagnostic and statistical manual of mental disorders. Am. Psychiatr. Assoc. 2013;21:591–643.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

