FAAH Inhibition Restores Early Life Stress-Induced Alterations in PFC microRNAs Associated with Depressive-Like Behavior in Male and Female Rats
- PMID: 36555739
- PMCID: PMC9782513
- DOI: 10.3390/ijms232416101
FAAH Inhibition Restores Early Life Stress-Induced Alterations in PFC microRNAs Associated with Depressive-Like Behavior in Male and Female Rats
Abstract
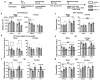
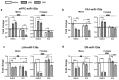
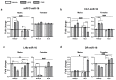
Early life stress (ELS) increases predisposition to depression. We compared the effects of treatment with the fatty acid amide hydrolase (FAAH) inhibitor URB597, and the selective serotonin reuptake inhibitor paroxetine, on ELS-induced depressive-like behavior and the expression of microRNAs (miRs) associated with depression in the medial prefrontal cortex (mPFC), hippocampal CA1 area, lateral habenula and dorsal raphe in rats. We also examined the mRNA expression of serotonergic (htr1a and slc6a4) and endocannabinoid (cnr1, cnr2 and faah) targets in the mPFC following ELS and pharmacological treatment. Adult males and females exposed to the 'Limited Bedding and Nesting' ELS paradigm demonstrated a depressive-like phenotype and late-adolescence URB597 treatment, but not paroxetine, reversed this phenotype. In the mPFC, ELS downregulated miR-16 in males and miR-135a in females and URB597 treatment restored this effect. In ELS females, the increase in cnr2 and decrease in faah mRNAs in the mPFC were reversed by URB597 treatment. We show for the first time that URB597 reversed ELS-induced mPFC downregulation in specific miRs and stress-related behaviors, suggesting a novel mechanism for the beneficial effects of FAAH inhibition. The differential effects of ELS and URB597 on males and females highlight the importance of developing sex-specific treatment approaches.
Keywords: URB597; cannabinoids; depression; early life stress; microRNAs.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
FAAH Inhibition Reverses Depressive-like Behavior and Sex-Specific Neuroinflammatory Alterations Induced by Early Life Stress.Cells. 2024 Nov 14;13(22):1881. doi: 10.3390/cells13221881. Cells. 2024. PMID: 39594629 Free PMC article.
-
Inhibition of Fatty Acid Amide Hydrolase (FAAH) During Adolescence and Exposure to Early Life Stress may Exacerbate Depression-like Behaviors in Male and Female Rats.Neuroscience. 2021 Feb 10;455:89-106. doi: 10.1016/j.neuroscience.2020.12.022. Epub 2020 Dec 25. Neuroscience. 2021. PMID: 33359656
-
Antidepressant-like effects of URB597 and JZL184 in male and female rats exposed to early life stress.Eur Neuropsychopharmacol. 2020 Oct;39:70-86. doi: 10.1016/j.euroneuro.2020.08.005. Epub 2020 Sep 2. Eur Neuropsychopharmacol. 2020. PMID: 32891517
-
Elevated Brain Fatty Acid Amide Hydrolase Induces Depressive-Like Phenotypes in Rodent Models: A Review.Int J Mol Sci. 2021 Jan 21;22(3):1047. doi: 10.3390/ijms22031047. Int J Mol Sci. 2021. PMID: 33494322 Free PMC article. Review.
-
Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597).CNS Drug Rev. 2006 Spring;12(1):21-38. doi: 10.1111/j.1527-3458.2006.00021.x. CNS Drug Rev. 2006. PMID: 16834756 Free PMC article. Review.
Cited by
-
Early life stress induces decreased expression of CB1R and FAAH and epigenetic changes in the medial prefrontal cortex of male rats.Front Cell Neurosci. 2024 Oct 22;18:1474992. doi: 10.3389/fncel.2024.1474992. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39503008 Free PMC article.
-
Epigenetic (re)programming of gene expression changes of CB1R and FAAH in the medial prefrontal cortex in response to early life and adolescence stress exposure.Front Cell Neurosci. 2023 Feb 22;17:1129946. doi: 10.3389/fncel.2023.1129946. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36909279 Free PMC article.
-
CBD enhances the cognitive score of adolescent rats prenatally exposed to THC and fine-tunes relevant effectors of hippocampal plasticity.Front Pharmacol. 2023 Jul 31;14:1237485. doi: 10.3389/fphar.2023.1237485. eCollection 2023. Front Pharmacol. 2023. PMID: 37583903 Free PMC article.
-
Decoding serotonin: the molecular symphony behind depression.Front Cell Neurosci. 2025 Apr 24;19:1572462. doi: 10.3389/fncel.2025.1572462. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40342516 Free PMC article. Review.
-
FAAH Inhibition Reverses Depressive-like Behavior and Sex-Specific Neuroinflammatory Alterations Induced by Early Life Stress.Cells. 2024 Nov 14;13(22):1881. doi: 10.3390/cells13221881. Cells. 2024. PMID: 39594629 Free PMC article.
References
-
- Nikkheslat N., McLaughlin A.P., Hastings C., Zajkowska Z., Nettis M.A., Mariani N., Enache D., Lombardo G., Pointon L., Cowen P.J. Childhood trauma, HPA axis activity and antidepressant response in patients with depression. Brain. Behav. Immun. 2020;87:229–237. doi: 10.1016/j.bbi.2019.11.024. - DOI - PMC - PubMed
-
- Xu X., Wu K., Ma X., Wang W., Wang H., Huang M., Luo L., Su C., Yuan T., Shi H. mGluR5-Mediated eCB Signaling in the Nucleus Accumbens Controls Vulnerability to Depressive-Like Behaviors and Pain After Chronic Social Defeat Stress. Mol. Neurobiol. 2021;58:4944–4958. doi: 10.1007/s12035-021-02469-9. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

