HIF-1α Causes LCMT1/PP2A Deficiency and Mediates Tau Hyperphosphorylation and Cognitive Dysfunction during Chronic Hypoxia
- PMID: 36555780
- PMCID: PMC9783654
- DOI: 10.3390/ijms232416140
HIF-1α Causes LCMT1/PP2A Deficiency and Mediates Tau Hyperphosphorylation and Cognitive Dysfunction during Chronic Hypoxia
Abstract
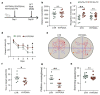
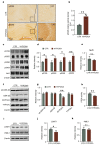
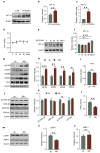
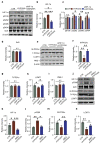
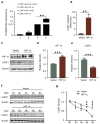
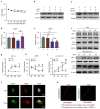
Chronic hypoxia is a risk factor for Alzheimer's disease (AD), and the neurofibrillary tangle (NFT) formed by hyperphosphorylated tau is one of the two major pathological changes in AD. However, the effect of chronic hypoxia on tau phosphorylation and its mechanism remains unclear. In this study, we investigated the role of HIF-1α (the functional subunit of hypoxia-inducible factor 1) in tau pathology. It was found that in Sprague-Dawley (SD) rats, global hypoxia (10% O2, 6 h per day) for one month induced cognitive impairments. Meanwhile it induced HIF-1α increase, tau hyperphosphorylation, and protein phosphatase 2A (PP2A) deficiency with leucine carboxyl methyltransferase 1(LCMT1, increasing PP2A activity) decrease in the rats' hippocampus. The results were replicated by hypoxic treatment in primary hippocampal neurons and C6/tau cells (rat C6 glioma cells stably expressing human full-length tau441). Conversely, HIF-1α silencing impeded the changes induced by hypoxia, both in primary neurons and SD rats. The result of dual luciferase assay proved that HIF-1α acted as a transcription factor of LCMT1. Unexpectedly, HIF-1α decreased the protein level of LCMT1. Further study uncovered that both overexpression of HIF-1α and hypoxia treatment resulted in a sizable degradation of LCMT1 via the autophagy--lysosomal pathway. Together, our data strongly indicated that chronic hypoxia upregulates HIF-1α, which obviously accelerated LCMT1 degradation, thus counteracting its transcriptional expression. The increase in HIF-1α decreases PP2A activity, finally resulting in tau hyperphosphorylation and cognitive dysfunction. Lowering HIF-1α in chronic hypoxia conditions may be useful in AD prevention.
Keywords: HIF-1α; chronic hypoxia; cognitive impairments; leucine carboxyl methyltransferase 1; protein phosphatase 2A; tau.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Leucine carboxyl methyltransferase 1 (LCMT1)-dependent methylation regulates the association of protein phosphatase 2A and Tau protein with plasma membrane microdomains in neuroblastoma cells.J Biol Chem. 2013 Sep 20;288(38):27396-27405. doi: 10.1074/jbc.M113.490102. Epub 2013 Aug 13. J Biol Chem. 2013. PMID: 23943618 Free PMC article.
-
Effects of Porphyromonas gingivalis and Its Underlying Mechanisms on Alzheimer-Like Tau Hyperphosphorylation in Sprague-Dawley Rats.J Mol Neurosci. 2021 Jan;71(1):89-100. doi: 10.1007/s12031-020-01629-1. Epub 2020 Jun 16. J Mol Neurosci. 2021. PMID: 32557144
-
Hypoxia modulates protein phosphatase 2A through HIF-1α dependent and independent mechanisms in human aortic smooth muscle cells and ventricular cardiomyocytes.Br J Pharmacol. 2019 Jun;176(11):1745-1763. doi: 10.1111/bph.14648. Epub 2019 Apr 22. Br J Pharmacol. 2019. PMID: 30825189 Free PMC article.
-
Role of microtubule-associated protein tau phosphorylation in Alzheimer's disease.J Huazhong Univ Sci Technolog Med Sci. 2017 Jun;37(3):307-312. doi: 10.1007/s11596-017-1732-x. Epub 2017 Jun 6. J Huazhong Univ Sci Technolog Med Sci. 2017. PMID: 28585125 Review.
-
Role of Hypoxia Inducible Factor-1α in Alzheimer's Disease.J Alzheimers Dis. 2021;80(3):949-961. doi: 10.3233/JAD-201448. J Alzheimers Dis. 2021. PMID: 33612545 Review.
Cited by
-
Hypoxia's Impact on Hippocampal Functional Connectivity: Insights from Resting-State fMRI Studies.Brain Sci. 2025 Jun 14;15(6):643. doi: 10.3390/brainsci15060643. Brain Sci. 2025. PMID: 40563813 Free PMC article. Review.
-
The Role of Oxygen Homeostasis and the HIF-1 Factor in the Development of Neurodegeneration.Int J Mol Sci. 2024 Apr 23;25(9):4581. doi: 10.3390/ijms25094581. Int J Mol Sci. 2024. PMID: 38731800 Free PMC article. Review.
-
Hypoxia-Inducible Factor 1α Affects Yak Oocyte Maturation and Early Embryonic Development by Regulating Autophagy.Antioxidants (Basel). 2024 Jul 14;13(7):840. doi: 10.3390/antiox13070840. Antioxidants (Basel). 2024. PMID: 39061908 Free PMC article.
-
Gypenoside IX restores Akt/GSK-3β pathway and alleviates Alzheimer's disease-like neuropathology and cognitive deficits.Aging (Albany NY). 2023 Dec 12;15(23):14172-14191. doi: 10.18632/aging.205295. Epub 2023 Dec 12. Aging (Albany NY). 2023. PMID: 38095632 Free PMC article.
-
Advancements in the investigation of the mechanisms underlying cognitive aging.Biogerontology. 2025 Aug 10;26(4):158. doi: 10.1007/s10522-025-10300-4. Biogerontology. 2025. PMID: 40783909 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

