Targeting TLR2/Rac1/cdc42/JNK Pathway to Reveal That Ruxolitinib Promotes Thrombocytopoiesis
- PMID: 36555781
- PMCID: PMC9787584
- DOI: 10.3390/ijms232416137
Targeting TLR2/Rac1/cdc42/JNK Pathway to Reveal That Ruxolitinib Promotes Thrombocytopoiesis
Abstract
Background: Thrombocytopenia has long been considered an important complication of chemotherapy and radiotherapy, which severely limits the effectiveness of cancer treatment and the overall survival of patients. However, clinical treatment options are extremely limited so far. Ruxolitinib is a potential candidate.
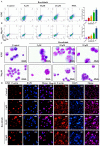
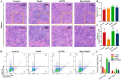
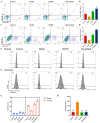
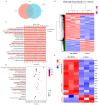
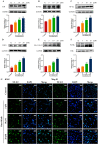
Methods: The impact of ruxolitinib on the differentiation and maturation of K562 and Meg-01 cells megakaryocytes (MKs) was examined by flow cytometry, Giemsa and Phalloidin staining. A mouse model of radiation-injured thrombocytopenia (RIT) was employed to evaluate the action of ruxolitinib on thrombocytopoiesis. Network pharmacology, molecular docking, drug affinity responsive target stability assay (DARTS), RNA sequencing, protein blotting and immunofluorescence analysis were applied to explore the targets and mechanisms of action of ruxolitinib.
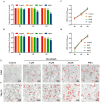
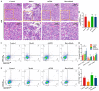
Results: Ruxolitinib can stimulate MK differentiation and maturation in a dose-dependent manner and accelerates recovery of MKs and thrombocytopoiesis in RIT mice. Biological targeting analysis showed that ruxolitinib binds directly to Toll Like Receptor 2 (TLR2) to activate Rac1/cdc42/JNK, and this action was shown to be blocked by C29, a specific inhibitor of TLR2.
Conclusions: Ruxolitinib was first identified to facilitate MK differentiation and thrombocytopoiesis, which may alleviate RIT. The potential mechanism of ruxolitinib was to promote MK differentiation via activating the Rac1/cdc42/JNK pathway through binding to TLR2.
Keywords: MK; TLR2/Rac1/cdc42/JNK; radiation; rusolitinib; thrombocytopoiesis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Harmine promotes megakaryocyte differentiation and thrombopoiesis by activating the Rac1/Cdc42/JNK pathway through a potential target of 5-HTR2A.Phytother Res. 2024 Nov;38(11):5134-5149. doi: 10.1002/ptr.8317. Epub 2024 Aug 17. Phytother Res. 2024. PMID: 39152726
-
Multimodal discovery of bavachinin A: A natural FLT3 agonist for treating thrombocytopenia.Phytomedicine. 2025 May;140:156597. doi: 10.1016/j.phymed.2025.156597. Epub 2025 Mar 1. Phytomedicine. 2025. PMID: 40058315
-
Novel chemical-structure TPOR agonist, TMEA, promotes megakaryocytes differentiation and thrombopoiesis via mTOR and ERK signalings.Phytomedicine. 2023 Feb;110:154637. doi: 10.1016/j.phymed.2022.154637. Epub 2022 Dec 28. Phytomedicine. 2023. PMID: 36610353
-
Oxymatrine, a novel TLR2 agonist, promotes megakaryopoiesis and thrombopoiesis through the STING/NF-κB pathway.J Pharm Anal. 2025 Jan;15(1):101054. doi: 10.1016/j.jpha.2024.101054. Epub 2024 Jul 23. J Pharm Anal. 2025. PMID: 39906691 Free PMC article.
-
The role of Rho GTPases' substrates Rac and Cdc42 in osteoclastogenesis and relevant natural medicinal products study.Biosci Rep. 2020 Jul 31;40(7):BSR20200407. doi: 10.1042/BSR20200407. Biosci Rep. 2020. PMID: 32578854 Free PMC article. Review.
Cited by
-
Efficacy of combined low-dose ruxolitinib and cyclosporine in murine immune bone marrow failure.Haematologica. 2024 May 1;109(5):1603-1607. doi: 10.3324/haematol.2023.284358. Haematologica. 2024. PMID: 38152039 Free PMC article. No abstract available.
-
Regulating mitochondrial oxidative phosphorylation and MAPK signaling: wedelolactone as a novel therapeutic for radiation-induced thrombocytopenia.Front Pharmacol. 2025 Apr 30;16:1508215. doi: 10.3389/fphar.2025.1508215. eCollection 2025. Front Pharmacol. 2025. PMID: 40371333 Free PMC article.
-
Ceramide-1-phosphate is a regulator of Golgi structure and is co-opted by the obligate intracellular bacterial pathogen Anaplasma phagocytophilum.mBio. 2024 Apr 10;15(4):e0029924. doi: 10.1128/mbio.00299-24. Epub 2024 Feb 28. mBio. 2024. PMID: 38415594 Free PMC article.
-
Cell Division Cycle 42 Improves Renal Functions, Fibrosis, Th1/Th17 Infiltration and Inflammation to Some Degree in Diabetic Nephropathy.Inflammation. 2025 Aug;48(4):1987-1997. doi: 10.1007/s10753-024-02169-1. Epub 2024 Nov 13. Inflammation. 2025. PMID: 39535664
References
-
- Tkaczynski E., Arulselvan A., Tkaczynski J., Avery S., Xiao L., Torok-Storb B., Abrams K., Rao N.V., Johnson G., Kennedy T.P., et al. 2-O, 3-O desulfated heparin mitigates murine chemotherapy- and radiation-induced thrombocytopenia. Blood. Adv. 2018;2:754–761. doi: 10.1182/bloodadvances.2017013672. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

