Oral Therapy for the Treatment of Transthyretin-Related Amyloid Cardiomyopathy
- PMID: 36555787
- PMCID: PMC9788438
- DOI: 10.3390/ijms232416145
Oral Therapy for the Treatment of Transthyretin-Related Amyloid Cardiomyopathy
Abstract
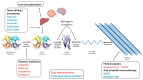
The care of systemic amyloidosis has improved dramatically due to improved awareness, accurate diagnostic tools, the development of powerful prognostic and companion biomarkers, and a continuous flow of innovative drugs, which translated into the blooming of phase 2/3 interventional studies for light chain (AL) and transthyretin (ATTR) amyloidosis. The unprecedented availability of effective drugs ignited great interest across various medical specialties, particularly among cardiologists who are now recognizing cardiac amyloidosis at an extraordinary pace. In all amyloidosis referral centers, we are observing a substantial increase in the prevalence of wild-type transthyretin (ATTRwt) cardiomyopathy, which is now becoming the most common form of cardiac amyloidosis. This review focuses on the oral drugs that have been recently introduced for the treatment of ATTR cardiac amyloidosis, for their ease of use in the clinic. They include both old repurposed drugs or fit-for-purpose designed compounds which bind and stabilize the TTR tetramer, thus reducing the formation of new amyloid fibrils, such as tafamidis, diflunisal, and acoramidis, as well as fibril disruptors which have the potential to promote the clearance of amyloid deposits, such as doxycycline. The development of novel therapies is based on the advances in the understanding of the molecular events underlying amyloid cardiomyopathy.
Keywords: ATTR amyloidosis; amyloid cardiomyopathy; amyloidosis; cardiac amyloidosis; emerging therapies; oral therapies; transthyretin.
Conflict of interest statement
M.N. is an inventor on a patent application unrelated to this work and received research funding from Oncopeptides, Gate Bioscience, and Pfizer. M.G. declares no conflict of interest. G.M. is a member of the Advisory Board of Jannsen and Pfizer.
Figures
References
-
- Maurer M.S., Bokhari S., Damy T., Dorbala S., Drachman B.M., Fontana M., Grogan M., Kristen A.V., Lousada I., Nativi-Nicolau J., et al. Expert Consensus Recommendations for the Suspicion and Diagnosis of Transthyretin Cardiac Amyloidosis. Circ. Heart Fail. 2019;12:e006075. doi: 10.1161/CIRCHEARTFAILURE.119.006075. - DOI - PMC - PubMed
-
- Mohamed-Salem L., Santos-Mateo J.J., Sanchez-Serna J., Hernandez-Vicente A., Reyes-Marle R., Castellon Sanchez M.I., Claver-Valderas M.A., Gonzalez-Vioque E., Haro-Del Moral F.J., Garcia-Pavia P., et al. Prevalence of wild type ATTR assessed as myocardial uptake in bone scan in the elderly population. Int. J. Cardiol. 2018;270:192–196. doi: 10.1016/j.ijcard.2018.06.006. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

