The Influence of Novel, Biocompatible, and Bioresorbable Poly(3-hydroxyoctanoate) Dressings on Wound Healing in Mice
- PMID: 36555799
- PMCID: PMC9785414
- DOI: 10.3390/ijms232416159
The Influence of Novel, Biocompatible, and Bioresorbable Poly(3-hydroxyoctanoate) Dressings on Wound Healing in Mice
Abstract
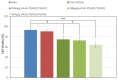
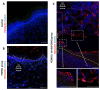
The human body's natural protective barrier, the skin, is exposed daily to minor or major mechanical trauma, which can compromise its integrity. Therefore, the search for new dressing materials that can offer new functionalisation is fully justified. In this work, the development of two new types of dressings based on poly(3-hydroxyoctanoate) (P(3HO)) is presented. One of the groups was supplemented with conjugates of an anti-inflammatory substance (diclofenac) that was covalently linked to oligomers of hydroxycarboxylic acids (Oli-dicP(3HO)). The novel dressings were prepared using the solvent casting/particulate leaching technique. To our knowledge, this is the first paper in which P(3HO)-based dressings were used in mice wound treatment. The results of our research confirm that dressings based on P(3HO) are safe, do not induce an inflammatory response, reduce the expression of pro-inflammatory cytokines, provide adequate wound moisture, support angiogenesis, and, thanks to their hydrophobic characteristics, provide an ideal protective barrier. Newly designed dressings containing Oli-dicP(3HO) can promote tissue regeneration by partially reducing the inflammation at the injury site. To conclude, the presented materials might be potential candidates as excellent dressings for wound treatment.
Keywords: CD68* macrophages; P(3HO); angiogenesis; bioresorption; dressing materials; poly(3-hydroxyoctanoate); polyhydroxyalkanoates; porous patches; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures














Similar articles
-
Physicochemical, structural and biological characterisation of poly(3-hydroxyoctanoate) supplemented with diclofenac acid conjugates - Harnessing the potential in the construction of materials for skin regeneration processes.Int J Biol Macromol. 2024 May;268(Pt 1):131476. doi: 10.1016/j.ijbiomac.2024.131476. Epub 2024 Apr 16. Int J Biol Macromol. 2024. PMID: 38614181
-
In vivo tests of a novel wound dressing based on biomaterials with tissue adhesion controlled through external stimuli.J Mater Sci Mater Med. 2011 May;22(5):1357-64. doi: 10.1007/s10856-011-4299-2. Epub 2011 Mar 25. J Mater Sci Mater Med. 2011. PMID: 21437637
-
Poly(3-hydroxyoctanoate), a promising new material for cardiac tissue engineering.J Tissue Eng Regen Med. 2018 Jan;12(1):e495-e512. doi: 10.1002/term.2318. Epub 2017 Aug 23. J Tissue Eng Regen Med. 2018. PMID: 27689781
-
Emerging Innovative Wound Dressings.Ann Biomed Eng. 2019 Mar;47(3):659-675. doi: 10.1007/s10439-018-02186-w. Epub 2018 Dec 12. Ann Biomed Eng. 2019. PMID: 30542783 Review.
-
Principles of Wound Dressings: A Review.Surg Technol Int. 2019 Nov 10;35:50-57. Surg Technol Int. 2019. PMID: 31480092 Review.
Cited by
-
Modulation of Macrophage Function by Bioactive Wound Dressings with an Emphasis on Extracellular Matrix-Based Scaffolds and Nanofibrous Composites.Pharmaceutics. 2023 Feb 28;15(3):794. doi: 10.3390/pharmaceutics15030794. Pharmaceutics. 2023. PMID: 36986655 Free PMC article. Review.
-
Fabrication and Characterization of a Stretchable Sodium Alginate Hydrogel Patch Combined with Silicon Nitride and Metalized Halloysite Nanotubes to Develop a Chronic Wound Healing Treatment.Int J Mol Sci. 2025 Feb 18;26(4):1734. doi: 10.3390/ijms26041734. Int J Mol Sci. 2025. PMID: 40004197 Free PMC article.
-
Transcriptome and metabolome analysis reveals PRV XJ delgE/gI/TK protects intracranially infected mice from death by regulating the inflammation.Front Microbiol. 2024 Mar 14;15:1374646. doi: 10.3389/fmicb.2024.1374646. eCollection 2024. Front Microbiol. 2024. PMID: 38550870 Free PMC article.
References
-
- Ghomi E.R., Khalili S., Khorasani S.N., Neisiany R.E. Wound dressings : Current advances and future directions. J. Appl. Polym. Sci. 2019;136:47738. doi: 10.1002/app.47738. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

