Lactobacillus gasseri LA806 Supplementation in Patients with Irritable Bowel Syndrome: A Multicenter Study
- PMID: 36556059
- PMCID: PMC9787120
- DOI: 10.3390/jcm11247446
Lactobacillus gasseri LA806 Supplementation in Patients with Irritable Bowel Syndrome: A Multicenter Study
Abstract
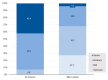
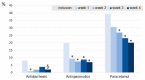
The potential benefits of Lactobacillus gasseri LA806 in IBS were previously identified in a comprehensive preclinical research program. The purpose of this multicenter study was to explore in real-life conditions changes in IBS symptoms and quality of life in patients receiving a 4-week supplementation with L. gasseri LA806. Altogether 119 patients meeting Rome IV criteria for IBS were included, of whom 118 received the supplement. The majority of patients (71.8% (95% CI 63.6−79.9%)) manifested a ≥30% decrease in abdominal pain at 4 weeks, the mean abdominal pain score diminishing by 54.2% (from 5.3 ± 2.2 to 2.2 ± 2.4, p < 0.0001). A statistically significant decrease in abdominal pain was seen as early as the first week. A decrease of ≥30% in both abdominal pain score and global IBS symptom score was attained in 61.5% of patients (95% CI 51.7−71.2%). The mean IBS-SSS score fell by 152 ± 112 points (p = 0.001), with symptoms being attenuated in 85% of patients (CGI-I). Supplementation led to a 10-fold decrease in the number of patients reporting severe IBS symptoms. The concomitant intake of antidiarrheals, antispasmodics and analgesics decreased and quality of life scores significantly improved. These preliminary results warrant confirmation by a randomized, placebo-controlled study that this study will allow a better design.
Keywords: abdominal pain; functional disease; irritable bowel syndrome; microbiota; probiotics; quality of life.
Conflict of interest statement
S.A.A. and C.G. are employed by Pileje Laboratoire. M.M., M.B. have no conflicts of interest to declare. J.S. has received consulting and lecture fees from PiLeJe Laboratoire. V.O. received consulting fees from PiLeJe Laboratoire.
Figures



References
-
- Sperber A.D., Bangdiwala S.I., Drossman D.A., Ghoshal U.C., Simren M., Tack J., Whitehead W.E., Dumitrascu D.L., Fang X., Fukudo S., et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation Global Study. Gastroenterology. 2021;160:99–114.e3. doi: 10.1053/j.gastro.2020.04.014. - DOI - PubMed
LinkOut - more resources
Full Text Sources

