The Transition from Cancer "omics" to "epi-omics" through Next- and Third-Generation Sequencing
- PMID: 36556377
- PMCID: PMC9785810
- DOI: 10.3390/life12122010
The Transition from Cancer "omics" to "epi-omics" through Next- and Third-Generation Sequencing
Abstract
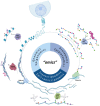
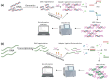
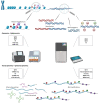
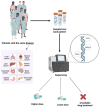
Deciphering cancer etiopathogenesis has proven to be an especially challenging task since the mechanisms that drive tumor development and progression are far from simple. An astonishing amount of research has revealed a wide spectrum of defects, including genomic abnormalities, epigenomic alterations, disturbance of gene transcription, as well as post-translational protein modifications, which cooperatively promote carcinogenesis. These findings suggest that the adoption of a multidimensional approach can provide a much more precise and comprehensive picture of the tumor landscape, hence serving as a powerful tool in cancer research and precision oncology. The introduction of next- and third-generation sequencing technologies paved the way for the decoding of genetic information and the elucidation of cancer-related cellular compounds and mechanisms. In the present review, we discuss the current and emerging applications of both generations of sequencing technologies, also referred to as massive parallel sequencing (MPS), in the fields of cancer genomics, transcriptomics and proteomics, as well as in the progressing realms of epi-omics. Finally, we provide a brief insight into the expanding scope of sequencing applications in personalized cancer medicine and pharmacogenomics.
Keywords: DNA methylation; RNA sequencing; cancer genomics; epigenomics; epiproteomics; epitranscriptomics; massive parallel sequencing; pharmacogenomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

