Hospital-Based Quasi-Experimental Study on Hydroxychloroquine Pre-Exposure Prophylaxis for COVID-19 in Healthcare Providers with Its Potential Side-Effects
- PMID: 36556412
- PMCID: PMC9786013
- DOI: 10.3390/life12122047
Hospital-Based Quasi-Experimental Study on Hydroxychloroquine Pre-Exposure Prophylaxis for COVID-19 in Healthcare Providers with Its Potential Side-Effects
Abstract
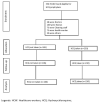
Considering that it has been more than 24 months since SARS-CoV-2 emerged, it is crucial to identify measures that prevent and control pathogen transmission in workplace settings. Our aim was to report results of a hospital-based program that delivered hydroxychloroquine (HCQ) tablets as COVID-19 prophylaxis to the frontline healthcare workers (HCW)s who cared for COVID-19 patients and to evaluate the efficacy of HCQ. Setting and participants: Quasi-experimental, controlled, single-center study. The included participants were doctors, nurses, health workers, cleaning staff, and non-healthcare supportive staff. The main outcome was contracting COVID-19 anytime during the period of taking the prophylaxis, confirmed by RT-PCR. A total of 336 participants, without any clinical evidence of COVID-19 and without any known contact with family members, were included in the trial; 230 were assigned to HCQ and 106 declined to take any drug. Results: Among the participants, 43 (18.7%) in the HCQ group and 11 (10.4%) participants in the control group developed COVID-19. For the evaluation of side effects, we evaluated 12-lead ECGs of both groups at the baseline and after 4 weeks to monitor QTc interval. A total of 91% (198 of 217) participants in the prophylaxis group and 92% (11 of 12) in the control group had a QTc < 45o msec, which is within normal limits. Conclusions: Although the number of symptomatic infections in health personnel was lower in the control group, the difference was not statistically significant. However, in the absence of any effective pre-exposure prophylaxis medicine for COVID-19, practicing proper infection prevention and control (IPC) and vaccination is the only way forward.
Keywords: COVID-19; corrected QT interval; healthcare workers; hydroxychloroquine; preexposure prophylaxis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Hydroxychloroquine plus personal protective equipment versus standard personal protective equipment alone for the prevention of COVID-19 infections among frontline healthcare workers: the HydrOxychloroquine Prophylaxis Evaluation(HOPE) trial: A structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Aug 31;21(1):754. doi: 10.1186/s13063-020-04679-3. Trials. 2020. PMID: 32867852 Free PMC article.
-
Efficacy of hydroxychloroquine for post-exposure prophylaxis to prevent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among adults exposed to coronavirus disease (COVID-19): a structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Jun 3;21(1):475. doi: 10.1186/s13063-020-04446-4. Trials. 2020. PMID: 32493478 Free PMC article.
-
Protecting Frontline Health Care Workers from COVID-19 with Hydroxychloroquine Pre-exposure Prophylaxis: A structured summary of a study protocol for a randomised placebo-controlled multisite trial in Toronto, Canada.Trials. 2020 Jul 14;21(1):647. doi: 10.1186/s13063-020-04577-8. Trials. 2020. PMID: 32665039 Free PMC article.
-
A systematic review of the prophylactic role of chloroquine and hydroxychloroquine in coronavirus disease-19 (COVID-19).Int J Rheum Dis. 2020 May;23(5):613-619. doi: 10.1111/1756-185X.13842. Epub 2020 Apr 27. Int J Rheum Dis. 2020. PMID: 32281213 Free PMC article.
-
Chloroquine and hydroxychloroquine for COVID-19: Perspectives on their failure in repurposing.J Clin Pharm Ther. 2021 Feb;46(1):17-27. doi: 10.1111/jcpt.13267. Epub 2020 Sep 27. J Clin Pharm Ther. 2021. PMID: 32981089 Free PMC article. Review.
References
-
- World Health Organization . Statement on the Second Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV) World Health Organization; Geneva, Switzerland: 2020.
-
- Director W. General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. World Health Organization; Geneva, Switzerland: 2020.
-
- World Health Organization . Coronavirus Disease 2019 (COVID-19): Situation Report. Volume 94 World Health Organization; Geneva, Switzerland: 2020.
-
- Miller M. 2019 Novel Coronavirus COVID-19 (2019-nCoV) Data Repository: Johns Hopkins University Center for Systems Science and Engineering. Bull.-Assoc. Can. Map Libr. Arch. 2020;164:47–51. doi: 10.15353/acmla.n164.1730. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous