Effect of Photodynamic Therapy with Chlorin e6 on Canine Tumors
- PMID: 36556469
- PMCID: PMC9782963
- DOI: 10.3390/life12122102
Effect of Photodynamic Therapy with Chlorin e6 on Canine Tumors
Abstract
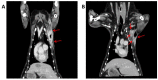
This work aims to prepare pure Chlorin e6 (Ce6) and establish Ce6-mediated photodynamic therapy (Ce6-PDT) as a better therapy option for canine tumors as well as mouse tumor models. Five dogs suffering from various cancers were treated with Ce6-PDT from one to several times. After receiving the Ce6 (2.5 mg/kg) for 3 h, tumors were illuminated superficially or interstitially with 660 nm light. Two dogs underwent Ce6-guided fluorescence imaging by photodynamic diagnosis (PDD). Cell proliferation and apoptosis were detected by the 4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay and western blot assay, respectively. Ce6-PDT efficacy was also determined using melanoma and pancreatic cancer mouse models. Two veterinary patients with mammary carcinoma and histiocytic sarcoma had their tumors significantly diminished and showed improved health after receiving Ce6-PDT. Moreover, in the cases of canine tumors, the adjunctive use of Ce6-PDD revealed cancers that were not visible with white light viewing and provided a visual contrast from surrounding tissues. Also, in vivo, Ce6-PDT remarkably reduced melanoma and pancreatic tumors in the mouse model. These findings could pave the way for a better understanding of the underlying processes of Ce6-PDT, making it an effective and safe candidate for use in human and veterinary applications to abolish various cancers.
Keywords: Ce6; PDD; PDT; canine tumors; photosensitizer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Miyake M., Nishimura N., Nakai Y., Fujii T., Owari T., Hori S., Morizawa Y., Gotoh D., Anai S., Torimoto K., et al. Photodynamic diagnosis-assisted transurethral resection using oral 5-aminolevulinic acid decreases the risk of repeated recurrence in non-muscle-invasive bladder cancer: A cumulative incidence analysis by the person-time method. Diagnostics. 2021;11:185. doi: 10.3390/diagnostics11020185. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

